Selective targeting of coagulation factor X Gla domain by negatively charged gold nanoparticles: a novel method for controlled antithrombotic therapy
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
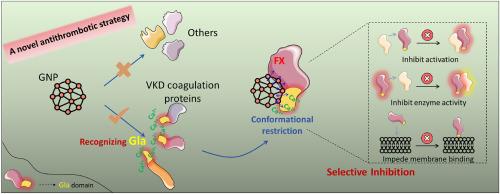
Venous thromboembolism (VTE) presents a significant global health burden due to its high incidence and potentially life-threatening complications. Although anticoagulants targeting vitamin K-dependent (VKD) factors, particularly factor X (FX), are widely employed, their efficacy is often limited by bleeding risks arising from off-target effects. Nanoparticle-based strategies, by contrast, enable precise and tunable modulation of protein activity through controlled adjustments in particle size, charge, and functionalization. In this work, we engineered negatively charged gold nanoparticles (GNPs) of defined sizes to selectively interact with the γ-carboxyglutamic acid (Gla) domain of VKD coagulation proteins. Using computational simulations, we systematically compared their binding conformations and affinities between GNPs and diverse VKD coagulation proteins, uncovering a size-dependent binding mechanism. This finding was subsequently validated through biochemical assays at both the molecular and cellular levels. Notably, GNPs with diameters of 2–3 nm demonstrated significantly higher affinity for FX compared to other VKD proteins, such as factor IX and protein C. This specific binding triggered substantial conformational changes in FX, diminishing its membrane-binding affinity. These structural alterations also reduced its enzymatic activity and impaired its activation efficiency within the coagulation cascade, thereby effectively attenuating the cascade by selectively modulating FX activity. Comprehensive in vitro coagulation assays and in vivo murine thrombosis models further validated that GNP treatment effectively prolonged coagulation time, demonstrating robust antithrombotic efficacy. Collectively, our results establish a novel nanoparticle-based therapeutic paradigm for targeting FX, offering an innovative and promising approach for enhancing the safety and efficacy of VTE prevention and management.
带负电荷的金纳米颗粒选择性靶向凝血因子X Gla结构域:一种控制抗血栓治疗的新方法
静脉血栓栓塞症(VTE)由于其高发病率和潜在危及生命的并发症而成为严重的全球健康负担。虽然针对维生素k依赖性因子(VKD),特别是X因子(FX)的抗凝剂被广泛使用,但其疗效往往受到脱靶效应引起的出血风险的限制。相比之下,基于纳米颗粒的策略可以通过控制颗粒大小、电荷和功能化来精确和可调地调节蛋白质活性。在这项工作中,我们设计了一定大小的带负电荷的金纳米颗粒(GNPs),以选择性地与VKD凝固蛋白的γ-羧谷氨酸(Gla)结构域相互作用。通过计算模拟,我们系统地比较了GNPs与不同VKD凝固蛋白之间的结合构象和亲和力,揭示了一种大小依赖的结合机制。这一发现随后通过分子和细胞水平的生化分析得到了验证。值得注意的是,与其他VKD蛋白(如因子IX和蛋白c)相比,直径为2-3 nm的GNPs对FX的亲和力明显更高,这种特异性结合引发了FX的实质性构象变化,降低了其膜结合亲和力。这些结构改变也降低了它的酶活性,损害了它在凝血级联中的激活效率,从而通过选择性调节FX活性有效地减弱了级联。综合体外凝血试验和小鼠体内血栓形成模型进一步验证了GNP治疗可有效延长凝血时间,显示出强大的抗血栓作用。总的来说,我们的研究结果建立了一种新的靶向FX的基于纳米颗粒的治疗范例,为提高静脉血栓栓塞预防和治疗的安全性和有效性提供了一种创新和有前途的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


