Dual–magnetically driven nanozymes for glioblastoma immunotherapy via magnetothermal and NIR–amplified ferroptosis and apoptosis
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
The hindrance posed by the blood-brain barrier (BBB) and the unique characteristics of the tumor microenvironment (TME) remain major challenges in glioblastoma (GBM) therapy. Here, we developed a dual-magnetically driven ultrasmall Mo0.2Fe2.8O4@CeOx/FA (MFCF) nanozyme exhibiting multienzyme catalytic activities for targeted synergistic therapy of GBM. This nanozyme demonstrated dual responsiveness to alternating magnetic fields (AMF) and static magnetic fields, synergized with folic acid (FA)–mediated molecular targeting to enhance BBB penetration and achieve high-precision GBM localization. Upon simultaneous exposure to AMF and near-infrared (NIR) laser irradiation, MFCF amplified reactive oxygen species (ROS) generation, depleted glutathione, and alleviated hypoxia through synergistic magnetothermal effects, type-II photodynamic therapy, and its intrinsic multienzyme catalytic activities, ultimately inducing both ferroptosis and apoptosis. Notably, this hybrid cell-death pathway triggered immunogenic cell death, promoting the proliferation and differentiation of T cells and thereby achieving systemic immune activation. Concurrently, it reprogrammed M2-polarized macrophages into pro-inflammatory M1 phenotypes, remodeling the immunosuppressive TME and enhancing antitumor immunotherapy. Furthermore, the excellent superparamagnetism of MFCF enabled T2-weighted magnetic resonance imaging (MRI)-guided treatment monitoring. Overall, this work presents a multifunctional nanoplatform that overcomes BBB and TME barriers to enable precise, immunomodulatory therapy for GBM.
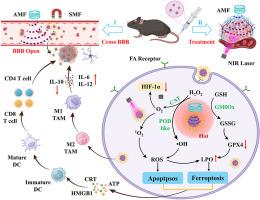
双磁驱动纳米酶通过磁热和nir扩增的铁凋亡和细胞凋亡免疫治疗胶质母细胞瘤
血脑屏障(BBB)造成的障碍和肿瘤微环境(TME)的独特特征仍然是胶质母细胞瘤(GBM)治疗的主要挑战。在这里,我们开发了一种双磁驱动的超小Mo0.2Fe2.8O4@CeOx/FA (MFCF)纳米酶,具有多酶催化活性,可用于GBM的靶向协同治疗。该纳米酶对交变磁场(AMF)和静态磁场具有双重响应性,与叶酸(FA)介导的分子靶向协同作用,增强血脑屏障穿透,实现高精度GBM定位。同时暴露于AMF和近红外(NIR)激光照射下,MFCF通过协同磁热效应、ii型光动力治疗及其内在的多酶催化活性,放大活性氧(ROS)的产生,耗尽谷胱甘肽,缓解缺氧,最终诱导铁凋亡和细胞凋亡。值得注意的是,这种混合细胞死亡途径触发免疫原性细胞死亡,促进T细胞的增殖和分化,从而实现全身免疫激活。同时,它将m2极化巨噬细胞重编程为促炎M1表型,重塑免疫抑制的TME,增强抗肿瘤免疫治疗。此外,MFCF优异的超顺磁性使t2加权磁共振成像(MRI)引导的治疗监测成为可能。总的来说,这项工作提出了一个多功能纳米平台,克服了血脑屏障和TME障碍,使GBM的精确免疫调节治疗成为可能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


