Green efficient approach for the synthesis of feruloylated acylglycerols using amberlyst-35 as a novel catalyst: optimization and function evaluation
IF 5.8
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
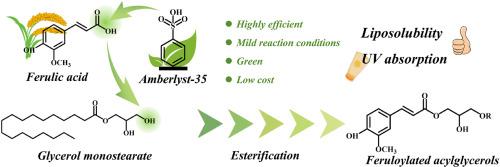
Ferulic acid (FA) is a natural phenolic compound with antioxidant and UV-absorbing properties. However, its poor lipophilicity limits its application in lipid-based systems. In this study, a sustainable and efficient approach was proposed for synthesizing lipophilic feruloylated acylglycerols (FAGs). The reaction was carried out through the esterification of FA with glycerides, catalyzed by the heterogeneous cation exchange resin Amberlyst-35. A range of reaction variables, including pressure, catalyst type, acyl acceptor, temperature, catalyst dosage, and substrate molar ratio, were systematically investigated. The results showed that A-35 and glycerol monostearate (GMS) were identified as the optimal catalyst and acyl acceptor, respectively. Response surface methodology was used to optimize the process. Under mild vacuum conditions (105 °C, 18 % catalyst dosage, FA:GMS = 1:7, reaction time 16 h), the FAGs yield could reach as high as 95.01 %. Kinetic analysis indicated Arrhenius-type behavior with an activation energy of 58.96 kJ/mol. The obtained FAGs exhibited strong UV absorption (λmax = 328 nm) and maintained more than 67 % photostability after 96 h of irradiation, which demonstrated its potential as a natural multifunctional additive. This study presents a practical method to produce lipophilic ferulic acid derivatives, helping develop sustainable ingredients for food and cosmetics.
以amberlyst-35为新型催化剂合成阿魏烯酰甘油的绿色高效方法:优化及功能评价
阿魏酸(FA)是一种天然酚类化合物,具有抗氧化和吸收紫外线的特性。然而,其较差的亲脂性限制了其在脂基体系中的应用。本研究提出了一种可持续、高效的合成亲脂阿魏酰化酰基甘油(FAGs)的方法。在非均相阳离子交换树脂Amberlyst-35的催化下,FA与甘油酯发生酯化反应。系统地研究了一系列反应变量,包括压力、催化剂类型、酰基受体、温度、催化剂用量和底物摩尔比。结果表明,A-35和单硬脂酸甘油(GMS)分别为最佳催化剂和酰基受体。采用响应面法对工艺进行优化。在温和真空条件下(105℃,18%催化剂用量,FA:GMS = 1:7,反应时间16 h), FAGs收率可达95.01%。动力学分析表明其为arrhenius型,活化能为58.96 kJ/mol。制备的FAGs具有较强的紫外吸收(λmax = 328 nm),辐照96 h后仍保持67%以上的光稳定性,具有作为天然多功能添加剂的潜力。本研究提出了一种生产亲脂阿魏酸衍生物的实用方法,有助于开发可持续发展的食品和化妆品原料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Sustainable Chemistry and Pharmacy
Environmental Science-Pollution
CiteScore
8.20
自引率
6.70%
发文量
274
审稿时长
37 days
期刊介绍:
Sustainable Chemistry and Pharmacy publishes research that is related to chemistry, pharmacy and sustainability science in a forward oriented manner. It provides a unique forum for the publication of innovative research on the intersection and overlap of chemistry and pharmacy on the one hand and sustainability on the other hand. This includes contributions related to increasing sustainability of chemistry and pharmaceutical science and industries itself as well as their products in relation to the contribution of these to sustainability itself. As an interdisciplinary and transdisciplinary journal it addresses all sustainability related issues along the life cycle of chemical and pharmaceutical products form resource related topics until the end of life of products. This includes not only natural science based approaches and issues but also from humanities, social science and economics as far as they are dealing with sustainability related to chemistry and pharmacy. Sustainable Chemistry and Pharmacy aims at bridging between disciplines as well as developing and developed countries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


