Selenium (VI) removal by volcanic ash material characterized by X-ray absorption spectroscopy
IF 1.8
4区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
This study investigates the potential use of volcanic ash (VA) material from Mount Cameroon as an adsorbent for the removal of selenium (VI) from contaminated solutions. Selenium-sorbed materials were synthesized via a batch adsorption method, and the resulting samples were characterized using scanning electron microscopy (SEM), energy-dispersive spectroscopy (EDS), and X-ray absorption spectroscopy (XAS). The results suggest that Se-sorbed VA material can adopt the local structure of CaSeO₃ and its chemical composition was associated with CaSeO₃, Na₂SeO₃, and Na₂SeO₄. The adsorption mechanisms were related to ion exchange, adsorption, and chemical reduction. Langmuir model was found to be suitable for the adsorption data indicating that the surface of VA adsorbent presents monolayer and homogeneous actives sites. XAS technique could be applied to track changes in oxidation state, resolve the adsorption mechanism, and identify the chemical species and local structure of the sorbed element in the adsorbent material.
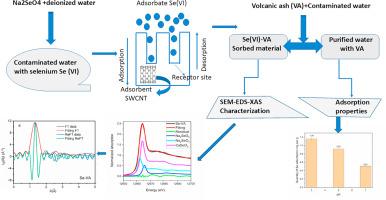
火山灰材料去除硒(VI)的x射线吸收光谱研究
本研究探讨了喀麦隆火山火山灰(VA)材料作为吸附剂从污染溶液中去除硒(VI)的潜在用途。采用间歇吸附法合成了硒吸附材料,并用扫描电镜(SEM)、能谱仪(EDS)和x射线吸收光谱(XAS)对样品进行了表征。结果表明,se吸附的VA材料可以采用CaSeO₃的局部结构,其化学成分与CaSeO₃、Na₂SeO₃和Na₂SeO₄相关联。吸附机理与离子交换、吸附和化学还原有关。Langmuir模型适用于吸附数据,表明VA吸附剂表面呈现单层均匀的活性位点。XAS技术可用于跟踪氧化态变化,解析吸附机理,鉴定吸附元素在吸附剂材料中的化学种类和局部结构。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Surface Science
化学-物理:凝聚态物理
CiteScore
3.30
自引率
5.30%
发文量
137
审稿时长
25 days
期刊介绍:
Surface Science is devoted to elucidating the fundamental aspects of chemistry and physics occurring at a wide range of surfaces and interfaces and to disseminating this knowledge fast. The journal welcomes a broad spectrum of topics, including but not limited to:
• model systems (e.g. in Ultra High Vacuum) under well-controlled reactive conditions
• nanoscale science and engineering, including manipulation of matter at the atomic/molecular scale and assembly phenomena
• reactivity of surfaces as related to various applied areas including heterogeneous catalysis, chemistry at electrified interfaces, and semiconductors functionalization
• phenomena at interfaces relevant to energy storage and conversion, and fuels production and utilization
• surface reactivity for environmental protection and pollution remediation
• interactions at surfaces of soft matter, including polymers and biomaterials.
Both experimental and theoretical work, including modeling, is within the scope of the journal. Work published in Surface Science reaches a wide readership, from chemistry and physics to biology and materials science and engineering, providing an excellent forum for cross-fertilization of ideas and broad dissemination of scientific discoveries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


