Theoretical investigate of an excited state intramolecular proton transfer mechanism of Br-3N-OH: Single or double
IF 4.7
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2025-09-24
DOI:10.1016/j.jphotochem.2025.116808
引用次数: 0
Abstract
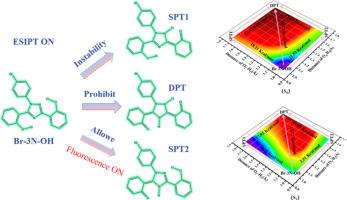
The dual-locked probe Br-3 N-2Et can be used to detect esterase in cells and endometrial cancer tissues. Br-3 N-2Et undergoes twisted intramolecular charge transfer (TICT) in the S1 state, resulting in fluorescence quenching. Its formation only requires overcoming an energy barrier of 6.37 kcal/mol, and the reverse energy barrier is higher, indicating that the TICT state stably exists. Upon reaction with esterase, Br-3 N-OH is generated. Hydrogen bond parameters, infrared vibrational spectra, and Reduced Density Gradient (RDG) analysis confirm the enhanced hydrogen bond strength in the S1 state. However, the excited-state double proton transfer (ESDPT) process is energetically unfavorable due to high energy barrier. The single proton transfer products (Br-3 N-OH-SPT1 and Br-3 N-OH-SPT2) can be generated. While Br-3 N-OH-SPT1 is unstable, the fluorescence emission peak of Br-3 N-OH-SPT2 (534 nm) closely matches with the experimental result (498 nm), indicating that the fluorescence mainly originates from Br-3 N-OH-SPT2. In summary, this work elucidates the quenching mechanism of Br-3 N-2Et and the luminescence mechanism of Br-3 N-OH, providing a theoretical guidance for designing analogous probes.
Br-3N-OH分子内激发态质子转移机制的理论研究:单或双
双锁紧探针Br-3 N-2Et可用于检测细胞和子宫内膜癌组织中的酯酶。br - 3n - 2et在S1态发生扭曲分子内电荷转移(TICT),导致荧光猝灭。它的形成只需要克服6.37 kcal/mol的能垒,逆能垒更高,表明TICT态稳定存在。与酯酶反应生成Br-3 N-OH。氢键参数、红外振动光谱和还原密度梯度(RDG)分析证实了S1态氢键强度的增强。然而,激发态双质子转移(ESDPT)过程由于高能势垒而在能量上是不利的。生成单质子转移产物Br-3 N-OH-SPT1和Br-3 N-OH-SPT2。而Br-3 N-OH-SPT1是不稳定的,Br-3 N-OH-SPT2的荧光发射峰(534 nm)与实验结果(498 nm)非常吻合,说明荧光主要来源于Br-3 N-OH-SPT2。综上所述,本研究阐明了br - 3n - 2et的猝灭机理和br - 3n - oh的发光机理,为类似探针的设计提供了理论指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


