CO2-to-methanol conversion over promoted Cu/ZnO/Al2O3 catalysts by Ga, Zr, and Co: A comprehensive DFT and experimental study
IF 7.6
Q1 ENERGY & FUELS
引用次数: 0
Abstract
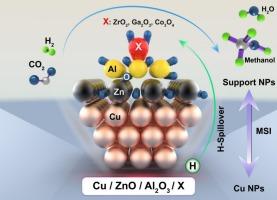
Methanol synthesis via CO2 hydrogenation over Cu/ZnO/Al2O3 catalysts is limited by low activity, deactivation, and the reverse water–gas shift reaction. In this study, the influence of Zr, Ga, and Co promoters was evaluated using both co-precipitation and impregnation methods. The catalysts were characterized by XRD, N2O chemisorption, H2-TPR, TGA, H2/CO2-TPD, and FE-SEM, while density functional theory (DFT) calculations provided qualitative insights into promoter effects on methoxy stabilization. Co-precipitated catalysts, particularly those containing Zr and Ga, showed improved copper dispersion, smaller crystallite sizes, and enhanced H2/CO2 adsorption. Under reaction conditions (235 °C, 50 bar, H2/CO2 = 3:1), Zr- and Ga-promoted catalysts achieved CO2 conversions of 42 % and 38 % with methanol selectivities of 98 % and 89 %, respectively. In contrast, Co-promoted catalysts exhibited higher CO and CH4 selectivities, indicating reduced suitability for methanol synthesis. These findings demonstrate that promoter type and synthesis route strongly influence the structural and catalytic properties of Cu-based catalysts for CO2 hydrogenation to methanol.
Ga、Zr和Co促进Cu/ZnO/Al2O3催化剂上co2转化为甲醇的综合DFT和实验研究
Cu/ZnO/Al2O3催化剂上CO2加氢合成甲醇存在活性低、失活和逆向水气转换反应等问题。在本研究中,采用共沉淀法和浸渍法评估了Zr、Ga和Co促进剂的影响。通过XRD、N2O化学吸附、H2- tpr、TGA、H2/CO2-TPD和FE-SEM对催化剂进行了表征,密度泛函理论(DFT)计算对促进剂对甲氧基稳定性的影响进行了定性分析。共沉淀催化剂,特别是含有Zr和Ga的催化剂,表现出更好的铜分散性、更小的晶粒尺寸和更强的H2/CO2吸附。在235℃,50 bar, H2/CO2 = 3:1的反应条件下,Zr和ga催化剂的CO2转化率分别为42%和38%,甲醇选择性分别为98%和89%。相比之下,共促进催化剂表现出更高的CO和CH4选择性,表明甲醇合成的适用性降低。这些结果表明,促进剂类型和合成路线对cu基催化剂的结构和催化性能有很大影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Energy Conversion and Management-X
Multiple-
CiteScore
8.80
自引率
3.20%
发文量
180
审稿时长
58 days
期刊介绍:
Energy Conversion and Management: X is the open access extension of the reputable journal Energy Conversion and Management, serving as a platform for interdisciplinary research on a wide array of critical energy subjects. The journal is dedicated to publishing original contributions and in-depth technical review articles that present groundbreaking research on topics spanning energy generation, utilization, conversion, storage, transmission, conservation, management, and sustainability.
The scope of Energy Conversion and Management: X encompasses various forms of energy, including mechanical, thermal, nuclear, chemical, electromagnetic, magnetic, and electric energy. It addresses all known energy resources, highlighting both conventional sources like fossil fuels and nuclear power, as well as renewable resources such as solar, biomass, hydro, wind, geothermal, and ocean energy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


