Organelle-targeted nanostructured lipid carriers: Strategic design, analytical validation, and translational insights for precision intracellular drug delivery
IF 4.9
3区 医学
Q1 PHARMACOLOGY & PHARMACY
Journal of Drug Delivery Science and Technology
Pub Date : 2025-10-01
DOI:10.1016/j.jddst.2025.107590
引用次数: 0
Abstract
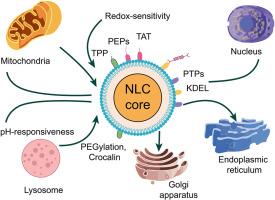
Precision intracellular drug delivery, enhanced payload capacity, physicochemical stability, and tunable release kinetics are the features offered by nanostructured lipid carriers (NLCs) as a next-generation drug delivery platform. Organelle-specific engineering and rational design of NLCs for targeted delivery to subcellular compartments, including the mitochondria, lysosomes, nucleus, endoplasmic reticulum (ER), and Golgi apparatus, are comprehensively discussed in this review. Strategic physicochemical modifications, such as particle size optimisation, surface functionalization with targeting ligands (e.g., TPP, TAT, NLS, KDEL), ceramide-mimetic components, and incorporation of pH or redox-responsive lipids, achieve organelle-specific localization, enabled by coupling chemistries like bio-orthogonal click reactions and EDC/NHS activation. High-resolution imaging modalities (CLSM, TEM, cryo-EM), co-localization quantification (Pearson's and Mander's coefficients), LC-MS/MS, FERT, FRAP, imaging flow cytometry, and differential scanning calorimetry (DSC) are used to validate targeting efficiency and formulation integrity analytically. Particle size, zeta potential, encapsulation efficiency, crystallinity, and release behavior are included in critical quality attributes (CQAs) that are optimised systematically using Design of Experiment (DoE) within a quality-by-design (QbD) framework. Physiologically based pharmacokinetics (PBPK) modelling and spatial lipidomics for assessing biodistribution and pharmacodynamic impact are used as preclinical translation, incorporating bioanalytical method validation. Endosomal entrapment, interspecies variability, and GMP-compliant challenges are the key translational and regulatory bottlenecks addressed in this review. Transformative potential for NLC-based precision medicine includes converging dual-organelle targeting, multi-stimuli responsiveness, emerging AI-guided design tools, and continuous manufacturing platforms. Redefining the landscape of intracellular therapeutics towards clinically viable organelle-targeted formulations through cutting-edge innovations is consolidated in this review.
细胞器靶向纳米结构脂质载体:精确细胞内药物传递的策略设计、分析验证和转化见解
精确的细胞内药物递送,增强的有效载荷能力,物理化学稳定性和可调的释放动力学是纳米结构脂质载体(nlc)作为下一代药物递送平台提供的特点。本文全面讨论了NLCs靶向递送至亚细胞区室(包括线粒体、溶酶体、细胞核、内质网和高尔基体)的细胞器特异性工程和合理设计。战略性的物理化学修饰,如粒径优化、靶向配体(如TPP、TAT、NLS、KDEL)的表面功能化、神经酰胺模拟成分,以及pH或氧化还原反应性脂质的掺入,通过耦合化学反应(如生物正交点击反应和EDC/NHS激活)实现细胞器特异性定位。高分辨率成像方式(CLSM, TEM, cro - em),共定位定量(Pearson's和Mander's系数),LC-MS/MS, FERT, FRAP,成像流式细胞术和差示扫描量热法(DSC)用于分析验证靶向效率和配方完整性。粒径、zeta电位、包封效率、结晶度和释放行为都包含在关键质量属性(cqa)中,这些属性在质量设计(QbD)框架中使用实验设计(DoE)进行系统优化。基于生理的药代动力学(PBPK)建模和用于评估生物分布和药效学影响的空间脂质组学用于临床前翻译,并结合生物分析方法验证。内体诱捕、种间变异和gmp合规挑战是本综述解决的关键转化和监管瓶颈。基于nlc的精准医疗的变革潜力包括融合双细胞器靶向、多刺激响应、新兴的人工智能引导设计工具和连续制造平台。重新定义的景观细胞内治疗朝着临床可行的细胞器靶向制剂通过前沿创新是巩固在这篇综述。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.00
自引率
8.00%
发文量
879
审稿时长
94 days
期刊介绍:
The Journal of Drug Delivery Science and Technology is an international journal devoted to drug delivery and pharmaceutical technology. The journal covers all innovative aspects of all pharmaceutical dosage forms and the most advanced research on controlled release, bioavailability and drug absorption, nanomedicines, gene delivery, tissue engineering, etc. Hot topics, related to manufacturing processes and quality control, are also welcomed.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


