Liposomal Formulations of Metal-CX5461 complexes: Copper-CX5461 complexation mediates CX5461 degradation while Zinc-CX5461 formulations are suitable for development
IF 4.9
3区 医学
Q1 PHARMACOLOGY & PHARMACY
Journal of Drug Delivery Science and Technology
Pub Date : 2025-09-30
DOI:10.1016/j.jddst.2025.107586
引用次数: 0
Abstract
Copper (Cu2+) ions have facilitated the encapsulation of CX5461 within liposomes and the formulations showed promising anticancer potential as judged by in-vivo studies. In our attempt to translate this formulation into a product suitable for use in formal pre-clinical safety pharmacology studies, we uncovered a problem. In this report, studies have demonstrated that Cu mediated CX5461 encapsulation within DSPC/Chol (55:45 mol%) and DMPC/Chol (55:45 mol%) liposomes can lead to instability of CX5461. Chromatographic analysis revealed that CX5461 degradation increases as the incubation temperature increased from 40 °C to 60 °C. The change in CX5461 structure results in the emergence of new peaks detected by HPLC and this is associated with a reduction in the parent drug. The rate of CX5461 degradation was influenced by incubation time and liposome lipid composition. When encapsulated in the DSPC/Chol:Cu(CX5461) liposome formulation, just 1 h of incubation at 40 °C or 60 °C resulted in greater than 50 % CX5461 degradation. This degradation was associated with a 1.7-fold and a 3.8-fold decrease in the CX5461 to liposomal lipid ratio within 1 h at 40 °C and 60 °C, respectively. When the CX5461 formulation was prepared using DMPC/Chol liposomes, CX5461 was less prone to degradation. Significant decrease in the CX5461 to liposomal lipid ratio for this formulation required 6 h of incubation at 40 °C or 60 °C. Surprisingly, even after complete degradation of CX5461, the degraded compounds were therapeutically as active as parent CX5461 based on in-vitro cytotoxicity studies. This suggested that the biologically active portion of CX5461 was maintained even after Cu-mediated degradation. Regardless, the extent and rate of degradation preclude the pharmaceutical development of the copper formulation as the active ingredient instability poses significant (perhaps insurmountable) challenges for chemistry, manufacturing, and control documentation. To overcome this limitation, we shifted to zinc as an alternative metal to complex CX-5461. Preliminary data demonstrate that Zn(CX5461) enables efficient liposomal encapsulation while preserving chemical stability.
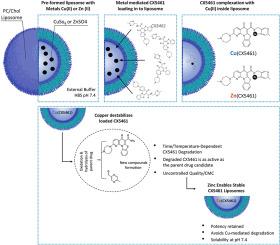
金属-CX5461配合物的脂质体制剂:铜-CX5461配合物介导CX5461的降解,而锌-CX5461制剂适合开发
铜(Cu2+)离子促进了CX5461在脂质体内的包封,体内研究表明,该制剂具有良好的抗癌潜力。在我们试图将该配方转化为适合用于正式临床前安全药理学研究的产品时,我们发现了一个问题。在本报告中,研究表明Cu介导的CX5461在dsc /Chol (55:45 mol%)和DMPC/Chol (55:45 mol%)脂质体中的包封可导致CX5461的不稳定性。色谱分析表明,CX5461的降解随孵育温度从40°C升高到60°C而增加。CX5461结构的变化导致HPLC检测到的新峰的出现,这与母体药物的减少有关。CX5461的降解速率受培养时间和脂质体组成的影响。当包封在dsc /Chol:Cu(CX5461)脂质体制剂中时,仅在40°C或60°C孵育1小时,导致CX5461降解大于50%。在40°C和60°C条件下,CX5461与脂质体的脂质比值在1小时内分别降低1.7倍和3.8倍。当使用DMPC/Chol脂质体制备CX5461制剂时,CX5461不易降解。在40°C或60°C孵育6小时后,CX5461与脂质体的脂质比例显著降低。令人惊讶的是,根据体外细胞毒性研究,即使在CX5461完全降解后,降解的化合物也具有与母体CX5461一样的治疗活性。这表明即使在cu介导的降解后,CX5461的生物活性部分仍保持不变。无论如何,降解的程度和速度阻碍了铜制剂的药物开发,因为活性成分的不稳定性对化学、制造和控制文件构成了重大(可能是不可克服的)挑战。为了克服这一限制,我们改用锌作为配合物CX-5461的替代金属。初步数据表明,Zn(CX5461)能够在保持化学稳定性的同时有效地包封脂质体。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.00
自引率
8.00%
发文量
879
审稿时长
94 days
期刊介绍:
The Journal of Drug Delivery Science and Technology is an international journal devoted to drug delivery and pharmaceutical technology. The journal covers all innovative aspects of all pharmaceutical dosage forms and the most advanced research on controlled release, bioavailability and drug absorption, nanomedicines, gene delivery, tissue engineering, etc. Hot topics, related to manufacturing processes and quality control, are also welcomed.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


