Control of host-guest complexation and release of PFAS with pH changes employing ionizable β-cyclodextrin derivatives
IF 7.7
Q2 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
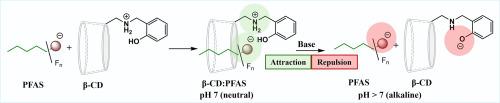
Poly- and perfluoroalkyl substances (PFAS) are organic pollutants whose widespread use, resistance to degradation, and adverse health effects have raised significant environmental and public health concerns. The ability of β-cyclodextrin (β-CD) to form strong inclusion complexes with PFAS has led to its incorporation in adsorbents for treating PFAS-contaminated water. Regenerating these PFAS-laden materials often remains challenging. We report herein the functionalization of β-CD with ionizable groups to control host-guest complexation with PFAS in response to pH changes. Adjusting the solution pH from neutral to alkaline led to a 56 to 98 % reduction in the binding constants (KCD:PFAS) of short- and long-chain PFAS with amino- and thiol-functionalized β-CDs. The observed reduction in binding constants under alkaline conditions is attributed to enhanced electrostatic repulsion between negatively charged functional groups attached to β-CD (host) and the anionic PFAS polar head group (guest). Incorporation of two pH-dependent ionizable functional groups, phenol and benzylamino, into β-CD to yield 6-(3-hydroxybenzylamino)-6-deoxy-β-cyclodextrin [(3-OH)BnNHβ-CD] enables the β-CD host to transition from a positive to a negative charge as the solution pH increases from neutral to alkaline. Consequently, (3-OH)BnNHβ-CD exhibits a pronounced pH-modulated complexation of PFOA, with an 88 % decrease in the association constant under alkaline conditions. The association constant for (3-OH)BnNHβ-CD with hexafluoropropylene oxide dimer acid (HFPO-DA), a branched perfluoroether carboxylic acid (PFECA), however, decreases by nearly 50 % under alkaline conditions compared to an 81 % and 98 % decrease observed for mono-thiol and mono-amino β-CDs, respectively. A 95 % decrease in binding in PFOA is observed for mono-thiol-β-CD, while heptakis-(6-mercapto-6-deoxy)-β-cyclodextrin, with seven ionizable thiol groups, leads to a modest 23 % decrease for complexation of PFOA with change from neutral to alkaline pH. Steric effects due to chain branching within PFAS in combination with size and number of substituents on the β-CD reduce the impact of pH effects on the complexation. This study demonstrates derivatization of β-CD with pH ionizable functional groups can be used to control the β-CD binding of PFAS as a possible strategy for the removal and recovery of PFAS from contaminated water streams.
利用可电离β-环糊精衍生物控制PFAS在pH变化下的主客体络合和释放
聚氟烷基和全氟烷基物质(PFAS)是有机污染物,其广泛使用、不易降解和对健康的不利影响已引起重大的环境和公共卫生关切。β-环糊精(β-CD)与PFAS形成强包合物的能力导致其掺入吸附剂中用于处理PFAS污染的水。再生这些含有pfas的材料通常仍然具有挑战性。我们在此报道了β-CD与可电离基团的功能化,以控制与PFAS的主客体络合,以响应pH变化。将溶液pH从中性调整为碱性,可使短链和长链PFAS与氨基和巯基功能化β-CDs的结合常数(KCD:PFAS)降低56%至98%。在碱性条件下观察到的结合常数的降低是由于与β-CD(宿主)连接的带负电荷官能团与阴离子PFAS极性头基(客体)之间的静电斥力增强。在β-CD中加入两个pH依赖的可电离官能团,苯酚和苄胺,生成6-(3-羟基苄胺)-6-脱氧-β-环糊精[(3-OH)BnNHβ-CD],使β-CD宿主随着溶液pH从中性到碱性的增加而从正电荷转变为负电荷。因此,(3-OH)BnNHβ-CD对PFOA具有明显的ph调节络合作用,在碱性条件下,缔合常数降低88%。然而,(3-OH)BnNHβ-CD与六氟环氧丙烷二聚酸(HFPO-DA)(一种支链全氟醚羧酸(peca))的结合常数在碱性条件下降低了近50%,而单硫醇和单氨基β- cd分别降低了81%和98%。单硫醇-β-CD与PFOA的结合降低了95%,而七烷基-(6-巯基-6-脱氧)-β-环糊精具有7个可电离的巯基,当pH值从中性变为碱性时,PFOA的配合度降低了23%。由于PFAS内链分支的位阻效应以及β-CD上取代基的大小和数量降低了pH值对配合度的影响。该研究表明,利用pH可电离官能团衍生化β-CD可用于控制PFAS的β-CD结合,作为从污染水流中去除和回收PFAS的可能策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of hazardous materials advances
Environmental Engineering
CiteScore
4.80
自引率
0.00%
发文量
0
审稿时长
50 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


