Efficient synthesis of l-DOPA in Escherichia coli via cofactor and enzyme engineering
IF 4.4
2区 生物学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
The global incidence of Parkinson's disease continues to rise. Levodopa (l-DOPA) is the core therapeutic drug, and efficient and sustainable production methods are needed. However, the complex metabolic pathways and the low catalytic efficiency of enzymes limit biosynthesis of l-DOPA in microorganisms. To address this issue, this study significantly enhanced the production efficiency of l-DOPA through a multi-dimensional, integrated metabolic and enzyme engineering approach. Firstly, the de novo synthesis pathway for l-DOPA was established through optimization of the promoter, ribosome-binding site (RBS), plasmid copy number, and tighly accurately regulating the expression level of key enzymes. Secondly, combined with metabonomic analysis, carbon metabolic flow was diverted, increasing the l-DOPA titer by 36.7 %. Glucose dehydrogenase (BmgdH) and gluconate kinase (gntK) were introduced to construct a cofactor regeneration system, which synergistically enhanced the supply of NADH and FADH2, increasing the l-DOPA conversion rate by 18 %. Next, the substrate tunnel of 4-hydroxyphenylacetic acid-3-monooxygenase subunit B (HpaB) was subjected to rational design, and mutant T292A significantly expanded the substrate channel, improved catalytic efficiency, and decreased l-tyrosine by 87 %. Finally, through the process optimization in a 5 L bioreactor (involving phased pH control and induction timing adjustment) achieved an l-DOPA titer of 60.73 g/L, the highest reported to date for de novo microbial synthesis. This research offers a novel approach for industrial biosynthesis of l-DOPA, and broadens engineering concepts for efficient synthesis of aromatic compounds.
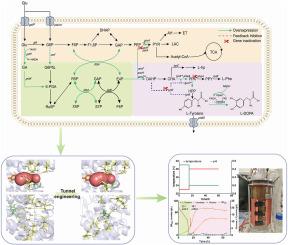
利用辅助因子和酶工程在大肠杆菌中高效合成左旋多巴
帕金森氏症的全球发病率持续上升。左旋多巴(l-DOPA)是核心治疗药物,需要高效、可持续的生产方法。然而,复杂的代谢途径和酶的低催化效率限制了微生物对左旋多巴的生物合成。为了解决这一问题,本研究通过多维、综合的代谢和酶工程方法,显著提高了左旋多巴的生产效率。首先,通过优化启动子、核糖体结合位点(RBS)、质粒拷贝数,严格准确调控关键酶的表达水平,建立l-DOPA从头合成途径。其次,结合代谢组学分析,改变了碳代谢流量,使左旋多巴滴度提高了36.7%。引入葡萄糖脱氢酶(BmgdH)和葡萄糖酸激酶(gntK)构建辅助因子再生体系,协同增强NADH和FADH2的供应,使l-DOPA转化率提高18%。接下来,对4-羟基苯基乙酸-3-单加氧酶亚基B (HpaB)的底物通道进行合理设计,突变体T292A显著扩大了底物通道,提高了催化效率,使l-酪氨酸降低了87%。最后,通过在5l生物反应器中进行工艺优化(包括分阶段pH控制和诱导时间调节),获得了60.73 g/L的L - dopa滴度,这是迄今为止报道的微生物从头合成的最高滴度。本研究为左旋多巴的工业生物合成提供了新的途径,拓宽了芳香族化合物高效合成的工程概念。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Synthetic and Systems Biotechnology
BIOTECHNOLOGY & APPLIED MICROBIOLOGY-
CiteScore
6.90
自引率
12.50%
发文量
90
审稿时长
67 days
期刊介绍:
Synthetic and Systems Biotechnology aims to promote the communication of original research in synthetic and systems biology, with strong emphasis on applications towards biotechnology. This journal is a quarterly peer-reviewed journal led by Editor-in-Chief Lixin Zhang. The journal publishes high-quality research; focusing on integrative approaches to enable the understanding and design of biological systems, and research to develop the application of systems and synthetic biology to natural systems. This journal will publish Articles, Short notes, Methods, Mini Reviews, Commentary and Conference reviews.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


