Dual-drug nanocarriers: Solid lipid nanoparticles and niosomes for enhanced denture disease treatment
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Nanotechnology-based carriers, such as solid lipid nanoparticles and niosomes, offer promising strategies to enhance the local delivery and efficacy of antimicrobial agents, including fluconazole and clindamycin, in periodontal therapy. Clindamycin-loaded niosomes were prepared using the thin-film hydration method with non-ionic surfactants and cholesterol, followed by hydration with clindamycin phosphate and probe sonication. Fluconazole-loaded solid lipid nanoparticles (FLZ-SLNs) were synthesized through high-shear homogenization and ultrasonication, alongside blank SLNs as controls. Transmission electron microscopy (TEM) confirmed spherical nanoparticles with sizes ranging from 96.9 to 181.1 nm for niosomes and 107.5–122.6 nm for SLNs. FTIR spectra revealed characteristic drug peaks (clindamycin: 3251, 1042, 565 cm−1; fluconazole: 3119, 674 cm−1) and shifts in O–H, C–H, and C–O bands upon drug encapsulation, confirming successful incorporation and interactions within carriers. UV–visible spectrophotometry calibration curves demonstrated strong linearity (R2 > 0.97) for precise quantification of the drug. Drug release studies revealed an initial rapid release (∼65 % within 200 min), followed by sustained release of up to 70 % over 24 h, with niosomes releasing faster initially. Zeta potentials ranged from −62.7 mV (drug-free SLNs) to −15.3 mV (drug-loaded SLNs), indicating altered nanoparticle stability post-drug loading. Antimicrobial assays demonstrated significantly enhanced inhibition zones for clindamycin-loaded niosomes (25–44 mm) and fluconazole-loaded SLNs (22–36 mm) against Staphylococcus aureus, Lacticaseibacillus casei, and Candida albicans, highlighting improved efficacy of these nanocarriers in dental soft-liner applications in comparison with the positive control.
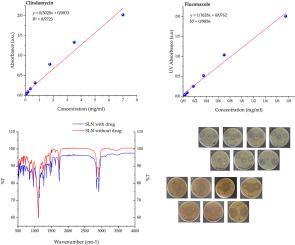
双药纳米载体:固体脂质纳米颗粒和纳米体增强义齿疾病治疗
基于纳米技术的载体,如固体脂质纳米颗粒和乳小体,在牙周治疗中提供了有希望的策略来增强抗微生物药物的局部递送和疗效,包括氟康唑和克林霉素。采用非离子表面活性剂和胆固醇的薄膜水化法制备了载克林霉素乳质体,然后与克林霉素磷酸水化,探针超声。通过高剪切均质和超声合成负载氟康唑的固体脂质纳米颗粒(FLZ-SLNs),并以空白SLNs为对照。透射电子显微镜(TEM)证实,纳米粒的尺寸为96.9 ~ 181.1 nm, sln的尺寸为107.5 ~ 122.6 nm。FTIR光谱显示了药物的特征峰(克林霉素:3251、1042、565 cm−1;氟康唑:3119、674 cm−1)和药物包封后O-H、C-H和C-O波段的移位,证实了药物在载体内的成功掺入和相互作用。紫外可见分光光度法校准曲线具有较强的线性(R2 > 0.97),可用于药物的精确定量。药物释放研究显示,最初的快速释放(200分钟内释放约65%),随后在24小时内持续释放高达70%,纳米体最初释放速度更快。Zeta电位范围从- 62.7 mV(无药SLNs)到- 15.3 mV(载药SLNs),表明载药后纳米颗粒的稳定性发生了变化。抗菌试验显示,载克林霉素的纳米粒体(25-44 mm)和载氟康唑的纳米粒体(22-36 mm)对金黄色葡萄球菌、干酪乳杆菌和白色念珠菌的抑制区显著增强,与阳性对照相比,这些纳米载体在牙科软衬中的应用效果有所提高。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


