Hydrothermal fabrication and OER electrocatalytic evaluation of MoS2/PANI nanocomposite
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
OER synthesis via water electrolysis by using effective electrocatalysts is an exciting phenomenon to be explored by researchers. Hydrogen is observed as clean energy means to meet commercial and domestic needs with minimum environmental hazards. In the proposed work we developed a nanocomposite comprised of flower shaped molybdenum disulfide (MoS2) and highly conductive polyaniline (PANI) by simple hydrothermal technique. The surface analysis and structural evaluation of organized composite material was carried with scanning electron microscopy and x-ray diffraction technique. The OER catalytic distinguishes of prepared electrode were studied through three electrode system within 1.0 M KOH electrolyte. The MoS2/PANI electrocatalyst showed low Tafel (51 mV/dec) and overpotential (242 mV) which is superior than Tafel (88 and 99 mV/dec) and overpotential (342 and 296 mV) of PANI and MoS2 respectively. The smaller resistance of composite (1.67 Ω) than pure MoS2 (2.14 Ω) can be attributed to enhanced ions mobility due to availability of π electronic structure and increased active sites in composite. Also, the composite electrode material showed excellent retention of electrocatalytic activity over 2300th LSV scan cycles. These remarkable features of prepared composite make it a suitable electrode material for energy generation where quality and cost both matters.
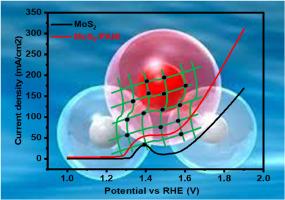
二硫化钼/聚苯胺纳米复合材料的水热制备及OER电催化评价
利用有效的电催化剂通过水电解法合成OER是一个令人兴奋的研究领域。氢被认为是一种清洁能源,以最小的环境危害满足商业和家庭需求。本文采用简单水热技术制备了一种由花状二硫化钼(MoS2)和高导电性聚苯胺(PANI)组成的纳米复合材料。采用扫描电镜和x射线衍射技术对组织好的复合材料进行了表面分析和结构评价。在1.0 M KOH电解液中,通过三电极体系研究了制备电极的OER催化鉴别。MoS2/PANI电催化剂表现出较低的过电压(51 mV/dec)和过电位(242 mV),优于PANI和MoS2的过电压(88 mV/dec)和过电位(342 mV/dec)。复合材料的电阻(1.67 Ω)比纯二硫化钼(2.14 Ω)小,这可以归因于π电子结构的可用性和复合材料中活性位点的增加,从而增强了离子的迁移率。此外,复合电极材料在2300次LSV扫描循环中表现出良好的电催化活性保持。所制备的复合材料的这些显著特征使其成为一种合适的电极材料,用于质量和成本都很重要的能源生产。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


