Effective recovering Pd(II) ions via tuning the Lewis basicity of nanofiber capture vacancy
IF 6.3
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
Recovery of palladium from spent catalysts is of great practical significance for the construction of ecological civilization and resource recycling. However, for environmentally friendly adsorption methods, designing specialized capture vacancies with high capacity and precise selectivity for Pd(II) ions remains a challenge. Herein, a salicylic acid-modified nanofiber (SANF), exhibiting specific spatial configuration and constructing a capture vacancy by “O![]() O” of hard bases, was designed and employed for recovering and separating palladium. The adsorption results indicated that the SANF exhibited a fast capture rate (reaching adsorption equilibrium within 60 min) and a large capture capacity (about 170 mg/g) for Pd(II) ions, and the capture process was exothermic and spontaneous. Additionally, the Lewis basicity of the capture vacancy after tuning better matches the Lewis acidity of Pd(II) ions, which achieves a high-selectivity separation of Pd(II) ions (selectivity coefficient for K(I), Na(I) Ca(II), Mg(II) and Al(III) ions are 1505.2, 10,536.7, 1128.9, 2634.2 and 2873.6, respectively). Practical applications showed that SANF was enabled to recover Pd(II) ions from spent catalyst leachate and achieved four time adsorption-desorption cycles, possessing some industrial promise. Furthermore, the matching mechanism between the Lewis basicity of the capture vacancy and the Lewis acidity of the Pd(II) ions was revealed through series characterization and theoretical calculations. Finally, it is proposed a Lewis basicity tuning strategy founded on a specific spatial structure, provides a new insight for the design and construction of a capture vacancy for Pd(II) ions in the future.
O” of hard bases, was designed and employed for recovering and separating palladium. The adsorption results indicated that the SANF exhibited a fast capture rate (reaching adsorption equilibrium within 60 min) and a large capture capacity (about 170 mg/g) for Pd(II) ions, and the capture process was exothermic and spontaneous. Additionally, the Lewis basicity of the capture vacancy after tuning better matches the Lewis acidity of Pd(II) ions, which achieves a high-selectivity separation of Pd(II) ions (selectivity coefficient for K(I), Na(I) Ca(II), Mg(II) and Al(III) ions are 1505.2, 10,536.7, 1128.9, 2634.2 and 2873.6, respectively). Practical applications showed that SANF was enabled to recover Pd(II) ions from spent catalyst leachate and achieved four time adsorption-desorption cycles, possessing some industrial promise. Furthermore, the matching mechanism between the Lewis basicity of the capture vacancy and the Lewis acidity of the Pd(II) ions was revealed through series characterization and theoretical calculations. Finally, it is proposed a Lewis basicity tuning strategy founded on a specific spatial structure, provides a new insight for the design and construction of a capture vacancy for Pd(II) ions in the future.
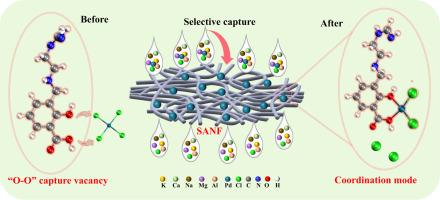
通过调整纳米纤维捕获空位的路易斯碱度,有效地回收Pd(II)离子
从废催化剂中回收钯对生态文明建设和资源循环利用具有重要的现实意义。然而,对于环境友好的吸附方法,设计具有高容量和精确选择性的Pd(II)离子的专门捕获空位仍然是一个挑战。本文设计了一种水杨酸修饰的纳米纤维(SANF),该纳米纤维具有特定的空间构型,并通过硬碱的“OO”结构构建捕获空位,用于钯的回收和分离。吸附结果表明,SANF对Pd(II)离子具有较快的捕获速率(60 min内达到吸附平衡)和较大的捕获量(约170 mg/g),且捕获过程为放热自发过程。此外,调整后捕获空位的Lewis碱度与Pd(II)离子的Lewis酸度匹配较好,实现了Pd(II)离子的高选择性分离(K(I)、Na(I)、Ca(II)、Mg(II)和Al(III)离子的选择性系数分别为1505.2、10536.7、1128.9、2634.2和2873.6)。实际应用表明,SANF能够从废催化剂渗滤液中回收Pd(II)离子,并实现4次吸附-解吸循环,具有一定的工业应用前景。通过系列表征和理论计算,揭示了俘获空位的Lewis碱度与Pd(II)离子的Lewis酸度之间的匹配机制。最后,提出了一种基于特定空间结构的Lewis碱度调整策略,为未来Pd(II)离子捕获空位的设计和构建提供了新的思路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Environmental Sciences-china
环境科学-环境科学
CiteScore
13.70
自引率
0.00%
发文量
6354
审稿时长
2.6 months
期刊介绍:
The Journal of Environmental Sciences is an international journal started in 1989. The journal is devoted to publish original, peer-reviewed research papers on main aspects of environmental sciences, such as environmental chemistry, environmental biology, ecology, geosciences and environmental physics. Appropriate subjects include basic and applied research on atmospheric, terrestrial and aquatic environments, pollution control and abatement technology, conservation of natural resources, environmental health and toxicology. Announcements of international environmental science meetings and other recent information are also included.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


