Stabilisation of a monoclonal antibody formulation in the presence of poloxamer 188 and related PEG/PPG systems
IF 4.9
3区 医学
Q1 PHARMACOLOGY & PHARMACY
Journal of Drug Delivery Science and Technology
Pub Date : 2025-09-23
DOI:10.1016/j.jddst.2025.107562
引用次数: 0
Abstract
Surfactants like poloxamer 188 are commonly used to stabilise antibody and other protein drug products. This study investigated the impact of poloxamer 188 on the stability of an antibody model protein in an aqueous buffered solution in comparison to mixtures composed of both polyethylene glycol and polypropylene glycol block polymers. The separate consideration of the block polymer units is important to understand the stabilising characteristics of poloxamer 188 and its degradation products. Therefore, interfacial stress was applied using a 48-hour shaking test to evaluate the stabilisation properties of the surfactants for an antibody. The effect of shaking was investigated by visual inspection, nephelometric turbidity measurement, size exclusion chromatography, and sub-visible particle formationvia backgrounded membrane imaging. Surface tension measurements revealed the impact of polyethylene glycol and polypropylene glycol on the air-water interface and showed that high surface activity is not the only decisive factor for protein stabilisation. It was determined that all excipients have the capacity to protect the antibody from protein particle formation to a certain extent. Polypropylene glycol performed better than polyethylene glycol due to a higher tendency to interact with the air-water interface. Surprisingly, mixtures of polyethylene glycol and polypropylene glycol, similar to their molecular weight ratio in poloxamer 188, led to comparable stabilisation properties to those of the triblock polymer poloxamer. This study indicates different stabilisation mechanisms for polyethylene glycol and polypropylene glycol combined in the poloxamer 188 molecule.
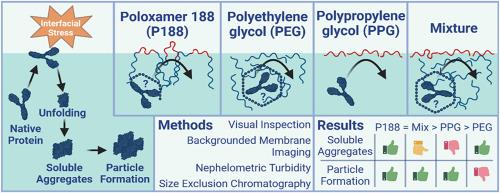
在泊洛沙姆188和相关PEG/PPG体系存在下单克隆抗体制剂的稳定性
像poloxam188这样的表面活性剂通常用于稳定抗体和其他蛋白质药物产品。本研究研究了poloxam188对抗体模型蛋白在水缓冲溶液中的稳定性的影响,并与由聚乙二醇和聚丙烯乙二醇嵌段聚合物组成的混合物进行了比较。单独考虑嵌段聚合物单元对于理解poloxam188及其降解产物的稳定特性是重要的。因此,使用48小时震动测试施加界面应力来评估表面活性剂对抗体的稳定性能。通过目视检查、浊度测量、粒径排除色谱和背景膜成像的亚可见颗粒形成来研究震动的影响。表面张力测量揭示了聚乙二醇和聚丙烯乙二醇对空气-水界面的影响,并表明高表面活性并不是蛋白质稳定的唯一决定性因素。确定所有赋形剂都有一定程度的保护抗体不受蛋白颗粒形成的能力。聚丙烯乙二醇比聚乙二醇表现得更好,因为它与空气-水界面的相互作用倾向更高。令人惊讶的是,聚乙二醇和聚丙烯乙二醇的混合物,与它们在poloxam188中的分子量比相似,导致了与三嵌段聚合物poloxam188相当的稳定性能。本研究揭示了聚乙二醇和聚丙烯乙二醇在泊洛沙姆188分子中的不同稳定机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.00
自引率
8.00%
发文量
879
审稿时长
94 days
期刊介绍:
The Journal of Drug Delivery Science and Technology is an international journal devoted to drug delivery and pharmaceutical technology. The journal covers all innovative aspects of all pharmaceutical dosage forms and the most advanced research on controlled release, bioavailability and drug absorption, nanomedicines, gene delivery, tissue engineering, etc. Hot topics, related to manufacturing processes and quality control, are also welcomed.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


