Evaluating the impact of lyophilization process parameters on mRNA encapsulated lipid nanoparticles using machine learning
IF 4.9
3区 医学
Q1 PHARMACOLOGY & PHARMACY
Journal of Drug Delivery Science and Technology
Pub Date : 2025-09-26
DOI:10.1016/j.jddst.2025.107573
引用次数: 0
Abstract
mRNA-encapsulated lipid nanoparticles (mRNA-LNPs) play a pivotal role in the mitigation of COVID-19. However, these vaccines are frequently accompanied by critical challenges such as a relatively short shelf life and the necessity for frozen storage. The development of lyophilized formulations has garnered significant interest in addressing these limitations, in turn enhancing the stability of mRNA-LNPs. Although lyophilization is a commonly employed manufacturing technique, systematic investigations of the relationship between its potential critical process parameters (pCPPs) and critical quality attributes (CQAs) of mRNA-LNPs remain scarce. In this study, we evaluated the effects of selected pCPPs (freezing rate, annealing temperature, and primary drying temperature) on various CQAs, including particle size, polydispersity index, mRNA and lipid content, encapsulation efficiency, RNA integrity, and in vitro activity. Among these attributes, only particle size exhibited a statistically significant variation across the investigated process parameters. Cryo-electron microscopy revealed that this observation was a consequence of bleb-like structures induced by lyophilization. Feature importance analysis based on a gradient boosting model identified freezing rate and annealing temperature as the most influential variables. The model further predicted that particle size was particularly sensitive to a freezing rate of approximately −0.3 °C/min, underscoring the necessity of precise control of freezing conditions to maintain product stability. These findings demonstrate that machine learning-based modelling can facilitate the quantitative evaluation of the effects of pCPPs on CQAs using a minimal number of experiments, thereby enabling a more comprehensive understanding of the mRNA-LNP manufacturing process and supporting the rational design of robust production strategies.
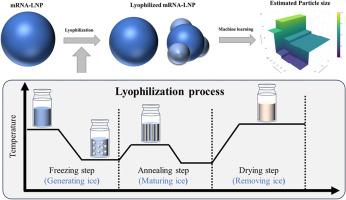
利用机器学习评估冻干工艺参数对mRNA包封脂质纳米颗粒的影响
mrna包封脂质纳米颗粒(mRNA-LNPs)在缓解COVID-19中发挥关键作用。然而,这些疫苗往往伴随着严峻的挑战,如保质期相对较短和需要冷冻储存。冻干制剂的发展在解决这些限制方面获得了极大的兴趣,从而提高了mRNA-LNPs的稳定性。尽管冻干是一种常用的制造技术,但对其潜在的关键工艺参数(pCPPs)和mRNA-LNPs的关键质量属性(cqa)之间关系的系统研究仍然很少。在这项研究中,我们评估了所选择的pCPPs(冷冻速率、退火温度和初级干燥温度)对各种cqa的影响,包括粒径、多分散性指数、mRNA和脂质含量、包封效率、RNA完整性和体外活性。在这些属性中,只有粒径在调查的工艺参数中表现出统计学上显著的变化。冷冻电子显微镜显示,这种观察结果是由冻干引起的泡状结构的结果。基于梯度增强模型的特征重要性分析发现,冻结速率和退火温度是影响最大的变量。该模型进一步预测,粒径对约- 0.3°C/min的冷冻速率特别敏感,强调了精确控制冷冻条件以保持产品稳定性的必要性。这些发现表明,基于机器学习的建模可以使用最少的实验数量来促进ppps对cqa的影响的定量评估,从而能够更全面地了解mRNA-LNP制造过程,并支持稳健生产策略的合理设计。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.00
自引率
8.00%
发文量
879
审稿时长
94 days
期刊介绍:
The Journal of Drug Delivery Science and Technology is an international journal devoted to drug delivery and pharmaceutical technology. The journal covers all innovative aspects of all pharmaceutical dosage forms and the most advanced research on controlled release, bioavailability and drug absorption, nanomedicines, gene delivery, tissue engineering, etc. Hot topics, related to manufacturing processes and quality control, are also welcomed.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


