Mechanism of boron removal and stabilization by in-situ formation of layered double hydroxides: Insight from spectroscopy and DFT studies
IF 6.3
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
A method for the effective in-situ formation of boron-containing Mg-Al layered double hydroxides (LDHs) was developed for boron removal and stabilization. The influence of the B/Al molar ratio and pH on the formation of Mg-Al-B–LDHs was investigated. Compared with the adsorption method, under a high B/Al ratio, the coprecipitation method increased the boron sorption density from 0.256 to 0.472 of Al. The Toxicity Characteristic Leaching Procedure showed that the boron-coprecipitated LDHs exhibited higher stability than the boron-adsorption LDHs. The synthesized LDH samples were characterized by X-ray diffraction, X-ray photoelectron spectroscopy, and solid-state 11B-NMR. The results showed that boron was effectively incorporated into the LDH structure for the coprecipitation method. Combined with the experimental results, a potential in-situ formation pathway for Mg-Al-B–LDHs was elucidated through density functional theory calculations. The boron tended to directly incorporate into the LDH structure in the coprecipitation method, whereas it was predominantly adsorbed on the LDH surface in the adsorption method. The adsorption energy demonstrated that boron preferentially bonded to Mg2+ sites on the surface. The mechanism of boron incorporation in the LDHs for the coprecipitation method involved precipitation of amorphous aluminum hydroxide, layered boehmite transformation, nucleation, and layer stacking. During these processes, boron formed complexes to enhance its stability. Residual boron underwent further reactions with the LDHs, including surface adsorption and ion exchange. These findings provide theoretical insight into the effective removal and long-term immobilization of boron in landfill leachate self-remediation processes.
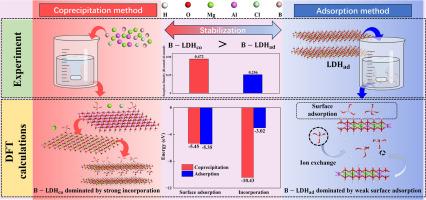
原位形成层状双氢氧化物去除硼和稳定硼的机理:来自光谱和DFT研究的见解
研究了一种原位生成含硼镁铝层状双氢氧化物(LDHs)的除硼稳定方法。考察了B/Al摩尔比和pH对Mg-Al-B-LDHs形成的影响。与吸附法相比,在高B/Al比下,共沉淀法将硼对Al的吸附密度从0.256提高到0.472。毒性特征浸出程序表明,硼共沉淀法的LDHs比硼吸附法的LDHs具有更高的稳定性。采用x射线衍射、x射线光电子能谱和固态11B-NMR对合成的LDH样品进行了表征。结果表明,在共沉淀法中,硼被有效地结合到LDH结构中。结合实验结果,通过密度泛函理论计算,阐明了Mg-Al-B-LDHs的潜在原位形成途径。在共沉淀法中,硼倾向于直接结合到LDH结构中,而在吸附法中,硼主要吸附在LDH表面。吸附能表明硼优先结合在表面的Mg2+位点上。共沉淀法中硼在LDHs中的掺入机制包括非晶态氢氧化铝的析出、层状薄水铝石转变、成核和层堆积。在这些过程中,硼形成配合物以增强其稳定性。残余硼与LDHs发生进一步的表面吸附和离子交换反应。这些发现为垃圾渗滤液自修复过程中硼的有效去除和长期固定化提供了理论见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Environmental Sciences-china
环境科学-环境科学
CiteScore
13.70
自引率
0.00%
发文量
6354
审稿时长
2.6 months
期刊介绍:
The Journal of Environmental Sciences is an international journal started in 1989. The journal is devoted to publish original, peer-reviewed research papers on main aspects of environmental sciences, such as environmental chemistry, environmental biology, ecology, geosciences and environmental physics. Appropriate subjects include basic and applied research on atmospheric, terrestrial and aquatic environments, pollution control and abatement technology, conservation of natural resources, environmental health and toxicology. Announcements of international environmental science meetings and other recent information are also included.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


