A comparison of charged and uncharged stabilizers on the loading of hydrophilic low and high molecular weight synthetic charged molecules in the LeciPlex® system
IF 4.9
3区 医学
Q1 PHARMACOLOGY & PHARMACY
Journal of Drug Delivery Science and Technology
Pub Date : 2025-09-19
DOI:10.1016/j.jddst.2025.107556
引用次数: 0
Abstract
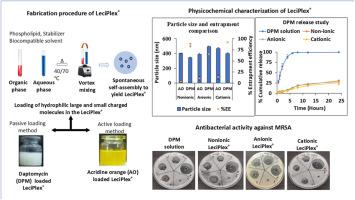
The present investigation aims to evaluate the loading of hydrophilic molecules with varying physicochemical properties in LeciPlex®. Polysorbate 80, taurocholate, and dioctadecyldimethylammonium bromide (DODAB) were used to prepare non-ionic, anionic and cationic LeciPlex® respectively, and their interactions with two model hydrophilic compounds differing in molecular weight, surface charge and structural features are explored. A negatively charged peptide, daptomycin was encapsulated via passive loading, while a positively charged amphipathic base, acridine orange required a remote loading. All formulations were characterized for particle size, zeta potential, and entrapment efficiency. Daptomycin and acridine orange LeciPlex® were further characterized by transmission electron microscopy (TEM) and evaluated for in vitro release profiles by dialysis bag method. In addition to the above characterizations, daptomycin-loaded LeciPlex® was further evaluated for thermal behavior using differential scanning calorimetry (DSC), antimicrobial efficacy via the resazurin assay, haemocompatibility and physicochemical stability. The type of stabilizer and phospholipid concentration directly influenced the particle size and entrapment of daptomycin LeciPlex®. The average particle size of daptomycin-loaded LeciPlex® was lower compared to the acridine orange-loaded LeciPlex®. Acridine orange entrapment (55–80 %) increased with cholesterol incorporation. TEM revealed uni or oligo lamellar vesicles. DSC confirmed the interaction of daptomycin with lipid in all three daptomycin-LeciPlex® systems. Both daptomycin and acridine orange LeciPlex® systems demonstrated sustained release profiles and improved stability. Daptomycin LeciPlex® showed comparable antimicrobial efficacy to that of the solution and exhibited minimal haemolysis. Present study demonstrated the successful encapsulation of structurally diverse hydrophilic molecules into LeciPlex® using a simplified and scalable approach.
LeciPlex®系统中负载亲水性低分子量和高分子量合成带电分子的带电和不带电稳定剂的比较
本研究旨在评价具有不同物理化学性质的亲水性分子在LeciPlex®中的负载。用聚山梨酯80、牛磺酸胆酸酯和二十八烷基二甲基溴化铵(DODAB)分别制备了非离子型、阴离子型和阳离子型LeciPlex®,并探讨了它们与两种不同分子量、表面电荷和结构特征的模型亲水性化合物的相互作用。带负电荷的多肽达托霉素通过被动负载封装,而带正电荷的两性碱吖啶橙则需要远程负载。对所有配方进行了粒径、zeta电位和捕集效率的表征。通过透射电镜(TEM)进一步表征达托霉素和吖啶橙LeciPlex®,并通过透析袋法评估其体外释放谱。除了上述表征外,还使用差示扫描量热法(DSC)进一步评估了负载达托霉素的LeciPlex®的热行为,通过resazurin试验评估了抗菌功效,血液相容性和理化稳定性。稳定剂的种类和磷脂浓度直接影响达托霉素的粒径和包裹度。负载达托霉素的LeciPlex®的平均粒径低于负载吖啶橙的LeciPlex®。吖啶橙夹持率(55 - 80%)随胆固醇掺入而增加。透射电镜显示单面或寡面片状囊泡。DSC证实了在所有三种达托霉素- leciplex®系统中,达托霉素与脂质相互作用。达托霉素和吖啶橙LeciPlex®系统均表现出缓释特性和提高的稳定性。达托霉素LeciPlex®显示出与溶液相当的抗菌功效,并表现出最小的溶血。本研究证明了使用简化和可扩展的方法成功地将结构多样的亲水性分子封装到LeciPlex®中。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.00
自引率
8.00%
发文量
879
审稿时长
94 days
期刊介绍:
The Journal of Drug Delivery Science and Technology is an international journal devoted to drug delivery and pharmaceutical technology. The journal covers all innovative aspects of all pharmaceutical dosage forms and the most advanced research on controlled release, bioavailability and drug absorption, nanomedicines, gene delivery, tissue engineering, etc. Hot topics, related to manufacturing processes and quality control, are also welcomed.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


