Cyanidin-3-O-galactoside attenuates Aβ aggregation and disintegrates preformed amyloid fibril
Q3 Pharmacology, Toxicology and Pharmaceutics
引用次数: 0
Abstract
Background
Amyloid-β (Aβ) fibril accumulation in the brain is a key feature of Alzheimer’s disease and a major therapeutic target. While the antibody aducanumab is currently the only approved agent for Aβ fibril elimination, no small-molecule alternative is available. Cyanidin-3-O-galactoside (Cy3Gal), a natural anthocyanin, has shown therapeutic potential, but its mechanism remains unclear.
Methods
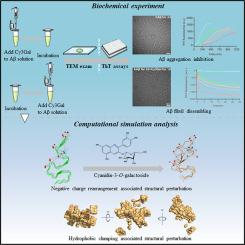
We evaluated Cy3Gal’s anti-amyloid effects using Thioflavin T (ThT) fluorescence assays and transmission electron microscopy (TEM) to assess its ability to slow down Aβ fibrillogenesis and disassemble existing fibrils. Molecular dynamics (MD) simulations were conducted to visualize the disassembly process, while microscale thermophoresis (MST) assessed Cy3Gal’s binding to monomeric and oligomeric Aβ. In vivo effects were tested using Caenorhabditis elegans CL2006 expressing human Aβ.
Results
Cy3Gal significantly attenuated Aβ aggregation and disassembled preformed fibrils, as confirmed by ThT reduction and TEM imaging. MD simulations revealed that Cy3Gal disrupts the fibril’s hydrophobic core and asparagine/glutamine ladder via electrostatic and hydrophobic interactions and MST data indicated a higher affinity for monomeric Aβ. Cy3Gal administration also alleviated Aβ-induced paralysis in C. elegans.
Conclusions
Cy3Gal exhibits dual activity in both attenuating Aβ fibrillogenesis and promoting fibril disassembly. Its mechanism involves structural disruption driven by flavylium–residue interactions and preferential monomer binding. These findings highlight Cy3Gal as a promising natural small-molecule candidate for Alzheimer’s therapy.
花青素-3- o -半乳糖苷减弱Aβ聚集并分解预先形成的淀粉样蛋白纤维
脑内淀粉样蛋白-β (a β)原纤维积累是阿尔茨海默病的一个关键特征,也是一个主要的治疗靶点。虽然抗体aducanumab是目前唯一被批准用于消除Aβ纤维的药物,但没有小分子替代药物可用。花青素-3- o -半乳糖苷(Cy3Gal)是一种天然花青素,已显示出治疗潜力,但其作用机制尚不清楚。方法采用硫黄素T (ThT)荧光法和透射电镜(TEM)检测Cy3Gal的抗淀粉样蛋白作用,以评估其减缓Aβ纤维形成和破坏现有原纤维的能力。分子动力学(MD)模拟显示了分解过程,而微尺度热电泳(MST)评估了Cy3Gal与单体和寡聚物Aβ的结合。用表达人Aβ的秀丽隐杆线虫CL2006检测其体内效应。结果ThT还原和TEM成像证实,scy3gal显著减弱了Aβ聚集,并破坏了预形成的原纤维。MD模拟表明,Cy3Gal通过静电和疏水相互作用破坏了原纤维的疏水核心和天冬酰胺/谷氨酰胺阶梯,MST数据表明其对单体a β具有更高的亲和力。给药Cy3Gal也能减轻a β诱导的秀丽隐杆线虫麻痹。结论scy3gal具有抑制Aβ纤维形成和促进纤维断裂的双重作用。其机制涉及由黄渣相互作用和优先单体结合驱动的结构破坏。这些发现突出了Cy3Gal作为阿尔茨海默病治疗的天然小分子候选者的前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytomedicine Plus
Medicine-Complementary and Alternative Medicine
CiteScore
3.70
自引率
0.00%
发文量
178
审稿时长
81 days
期刊介绍:
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


