Vitamin D3 encapsulated in polymeric nanoparticles to dampen the pro-inflammatory immune response
IF 3.6
Q2 IMMUNOLOGY
引用次数: 0
Abstract
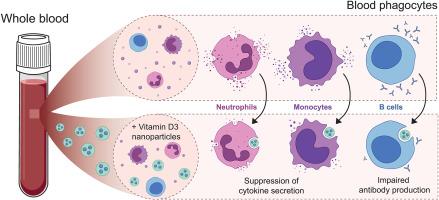
1α25-dihydroxyvitamin D3, the active metabolite of vitamin D3 (VD3), is a modulator of inflammation well-known for its ability to promote anti-inflammatory and tolerogenic immune responses. It is therefore an attractive agent for the attenuation of inflammatory responses and the development of tolerogenic immunity in autoimmune diseases. To overcome VD3 toxicity and enhance its in vivo performance, nanoparticles (NPs) have emerged as a promising delivery platform. Therefore, in this study, we have developed VD3-loaded polymeric nanoparticles (VD3-NPs) as a therapeutical strategy for the treatment of autoimmune disorders. We demonstrate that VD3-NPs could successfully be generated and that they significantly inhibit secretion of IL-6, IL-10, IL-23, and TNFα in human whole blood cultures. We observed that poly(lactic-co-glycolic acid) (PLGA) NPs are efficiently taken up by neutrophils, monocytes and B cells, prompting further investigation into the effect of VD3-NPs on these subsets. Investigation into each of the immune cell subsets demonstrated that the VD3-NPs were able inhibit cytokine secretion by both monocytes and neutrophils. Moreover, VD3-NPs induced a tolerogenic phenotype in monocytes. In B cells, we observed that VD3-NPs impaired in vitro plasma B cell differentiation and suppressed antibody production. Together, our results validate for the first time in primary human cells the therapeutic potential of VD3 encapsulated in PLGA NPs, posing an attractive strategy for the treatment of autoimmune diseases.
维生素D3封装在聚合纳米颗粒中,以抑制促炎免疫反应
1α25-二羟基维生素D3是维生素D3 (VD3)的活性代谢物,是一种炎症调节剂,以其促进抗炎和耐受性免疫反应的能力而闻名。因此,对于自身免疫性疾病的炎症反应的衰减和耐受性免疫的发展,它是一种有吸引力的药物。为了克服VD3的毒性并提高其在体内的性能,纳米颗粒(NPs)已成为一种有前途的给药平台。因此,在这项研究中,我们开发了装载vd3的聚合物纳米颗粒(VD3-NPs)作为治疗自身免疫性疾病的治疗策略。我们证明了VD3-NPs可以成功生成,并且它们可以显著抑制人全血培养中IL-6、IL-10、IL-23和tnf - α的分泌。我们观察到聚乳酸-羟基乙酸(PLGA) NPs被中性粒细胞、单核细胞和B细胞有效吸收,这促使我们进一步研究VD3-NPs对这些亚群的影响。对每个免疫细胞亚群的研究表明,VD3-NPs能够抑制单核细胞和中性粒细胞的细胞因子分泌。此外,VD3-NPs在单核细胞中诱导耐受性表型。在B细胞中,我们观察到VD3-NPs损害了体外血浆B细胞的分化并抑制了抗体的产生。总之,我们的研究结果首次在人类原代细胞中验证了包裹在PLGA NPs中的VD3的治疗潜力,为治疗自身免疫性疾病提出了一种有吸引力的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Translational Autoimmunity
Medicine-Immunology and Allergy
CiteScore
7.80
自引率
2.60%
发文量
33
审稿时长
55 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


