Pore size control of ordered mesoporous carbon by amino acid addition in solvent-free soft-template preparation
IF 4.7
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
The solvent-free soft template method is expected to be a promising scale-up preparation method for ordered mesoporous carbons because of its simple procedure. In order to expand the application possibilities of ordered mesoporous carbons prepared by solvent-free soft template method, we have developed the controlling method for pore size and structure by tuning the packing parameter of self-assembled carbon precursor composites. The self-assembled composites are composed of hydrophobic cores of triblock copolymers and hydrophilic shells of resorcinol interacted with hydrophilic tails of triblock copolymers. In this study, L-glutamic acid and L-cysteine with different hydrophilicity/hydrophobicity were added to the precursors. The addition of hydrophobic L-cysteine increased the packing parameter (the g value), which transformed the ordered structure from 1D channel-like pore structure to wormhole-like 3D pore structure. The mesopore size was decreased because the L-cysteine in the hydrophobic core is carbonized and incorporated into the pore wall. On the other hand, the addition of hydrophilic L-glutamic acid reduced the packing parameter (the g value) and the core-shell structure was no longer maintained. As a result, aggregates of uniform nanoparticles were formed. The size of mesopores formed between nanoparticles is larger than the above ordered carbons. The addition of amino acids appropriately increased the nitrogen content of carbon materials. When used as electrodes, mesoporous N-containing carbon with a larger pore size and 1D structure exhibited high double-layer capacitor performance. In summary, this study proposed a new method for controlling the pore size of ordered mesoporous carbons in solvent-free soft-template method.
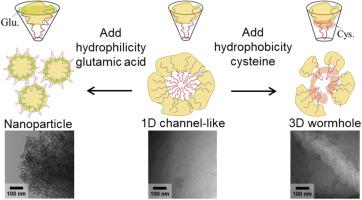
氨基酸加入对无溶剂软模板制备中有序介孔碳孔径的控制
无溶剂软模板法制备有序介孔碳因其工艺简单,有望成为一种有前途的规模化制备方法。为了拓展无溶剂软模板法制备有序介孔碳的应用前景,研究了通过调整自组装碳前驱体复合材料的填充参数来控制其孔径和结构的方法。自组装复合材料由三嵌段共聚物的疏水核和间苯二酚的亲水壳与三嵌段共聚物的亲水尾相互作用组成。本研究在前体中加入了亲疏水性不同的l -谷氨酸和l -半胱氨酸。疏水性l-半胱氨酸的加入增加了填料参数(g值),使有序结构由一维通道状孔隙结构转变为虫孔状三维孔隙结构。由于疏水核中的l -半胱氨酸碳化并掺入孔壁,介孔尺寸减小。另一方面,亲水性l -谷氨酸的加入降低了包装参数(g值),不再保持核壳结构。结果,形成了均匀的纳米颗粒聚集体。纳米颗粒之间形成的介孔尺寸大于上述有序碳。氨基酸的添加适当提高了碳材料的含氮量。当用作电极时,具有较大孔径和一维结构的介孔含氮碳具有较高的双层电容器性能。综上所述,本研究提出了一种无溶剂软模板法控制有序介孔碳孔径的新方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


