Effect of pore morphology on adsorption of methylene blue and phenol in micro-mesoporous carbons
IF 4.7
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Micro–mesoporous carbonaceous adsorbents were synthesized via polycondensation of resorcinol and furfural using soft-templating approach. Four templated samples were prepared with non-ionic surfactants Pluronic F-127 and P-123; a non-templated material was also synthesized for comparison. TEM showed that three templated carbons exhibited wormlike mesopores, while one had an ordered mesoporous structure. Nitrogen sorption and thermoporometry analyses revealed mesopore volumes from 0.19 to 0.40 cm3 g−1 and micropore volumes from 0.19 to 0.27 cm3 g−1. Higher template amount (5 g) resulted in broader mesopore distributions (up ∼15–20 nm) than lower amount (2 g, up ∼10 nm). All templated samples exhibited enhanced microporosity relative to non-templated reference, attributed to improved mesopore accessibility. Adsorption kinetics of phenol and methylene blue from aqueous solutions were evaluated using four kinetic models. The pseudo-second-order model provided the best fit based on Akaike information criterion. Mesopore arrangement (ordered vs. wormlike) significantly influenced adsorption behaviour. For phenol, adsorption capacities were similar across all templated samples, reflecting its preferential uptake in micropores, yet the ordered mesoporous carbon exhibited a notably lower rate constant, suggesting hindered diffusion due to less interconnected mesopores. For methylene blue, which preferentially adsorbs in mesopores and larger micropores, reduced adsorbed amount was observed in the ordered sample. This suppression correlated with limited water intrusion into mesopores, as confirmed by TPM, emphasizing the importance of surface wettability. These results demonstrate that both mesopore morphology and hydrophilicity, in addition to pore size, play a crucial role in governing adsorption performance of carbon materials in aqueous media.
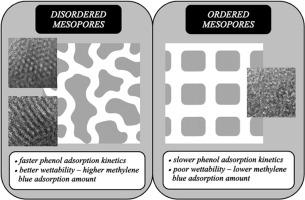
微介孔碳对亚甲基蓝和苯酚吸附性能的影响
以间苯二酚和糠醛为原料,采用软模板法缩聚合成了微介孔碳质吸附剂。用非离子表面活性剂Pluronic F-127和P-123制备了4个模板样品;还合成了一种非模板材料进行比较。TEM结果表明,3个模板炭具有蠕虫状介孔结构,1个模板炭具有有序介孔结构。氮气吸附和热容分析表明,中孔体积为0.19 ~ 0.40 cm3 g - 1,微孔体积为0.19 ~ 0.27 cm3 g - 1。较高的模板量(5 g)比较低的模板量(2 g, 10 nm)导致更宽的介孔分布(高达15-20 nm)。所有模板样品相对于非模板样品表现出增强的微孔隙度,这归因于改进的介孔可及性。采用四种动力学模型对苯酚和亚甲基蓝在水溶液中的吸附动力学进行了评价。伪二阶模型基于赤池信息准则提供了最优拟合。介孔排列(有序与蠕虫状)显著影响吸附行为。对于苯酚,所有模板样品的吸附能力相似,反映了其在微孔中的优先吸收,而有序介孔碳表现出明显较低的速率常数,表明由于介孔互连较少而阻碍了扩散。亚甲基蓝优先吸附于中孔和较大的微孔中,有序样品的吸附量减少。正如TPM所证实的那样,这种抑制与有限的水侵入介孔有关,强调了表面润湿性的重要性。这些结果表明,中孔形态和亲水性以及孔径大小对碳材料在水介质中的吸附性能起着至关重要的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


