Preparation of anodic catalysts via in situ exsolution of Pt nanoparticles for a methane oxidation enhanced SOEC process
IF 3.7
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
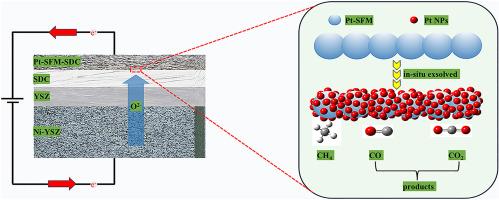
Introducing methane at the anode side of a solid oxide electrolysis cell (SOEC) has been proven to effectively suppress the oxygen evolution reaction (OER), thereby enabling hydrogen production at significantly lower voltages. In this work, a double perovskite oxide, Sr2Fe1.4Pt0.1Mo0.5O6-δ (abbreviated as Pt-SFM), was successfully synthesized by a liquid-phase method and employed as both an electronic conductor and a catalyst for methane oxidation at the SOEC anode. Following high-temperature treatment under a reducing atmosphere, platinum (Pt) nanoparticles were exsolved from the perovskite lattice and uniformly dispersed on the oxide surface. These exsolved Pt nanoparticles act as highly active sites for methane adsorption and oxidation. Electrochemical performance tests were conducted at 1123.15 K, and the results demonstrated that the Pt-SFM cell treated for 20 h (Pt-SFM 20 h) achieved a current density of 0.85 A·cm−2 at an applied voltage of 1.40 V. This performance corresponds to a 102.4% enhancement compared to the undoped SFM 20 h cell. The superior performance is attributed to the presence of exsolved Pt, which significantly improves the catalyst's ability to adsorb and dissociate methane molecules. Electrochemical impedance spectroscopy (EIS) analysis under open-circuit conditions revealed that the polarization impedance of the Pt-SFM 20 h cell was 1.25 Ω·cm2, which is 49.2% lower than that of the SFM 20 h cell. Furthermore, a 45-h long-term stability test showed that the Pt-SFM 20 h cell maintained a stable performance, with a low voltage degradation rate of only 0.67 mV·h−1.
原位外溶Pt纳米颗粒制备甲烷氧化强化SOEC阳极催化剂
在固体氧化物电解电池(SOEC)的阳极侧引入甲烷已被证明可以有效抑制析氧反应(OER),从而在显著降低的电压下制氢。本文采用液相法成功合成了一种双钙钛矿氧化物Sr2Fe1.4Pt0.1Mo0.5O6-δ(简称Pt-SFM),并将其作为SOEC阳极甲烷氧化的电子导体和催化剂。在还原气氛下进行高温处理后,铂纳米颗粒从钙钛矿晶格中析出,均匀地分散在氧化物表面。这些溶解的铂纳米颗粒作为甲烷吸附和氧化的高活性位点。在1123.15 K下进行电化学性能测试,结果表明,在1.40 V电压下,Pt-SFM电池处理20 h (Pt-SFM 20 h)的电流密度为0.85 a·cm−2。与未掺杂的SFM 20 h电池相比,该性能提高了102.4%。这种优异的性能归功于Pt的存在,它显著提高了催化剂对甲烷分子的吸附和解离能力。开路条件下电化学阻抗谱(EIS)分析表明,Pt-SFM 20 h电池的极化阻抗为1.25 Ω·cm2,比SFM 20 h电池的极化阻抗低49.2%。此外,45 h的长期稳定性测试表明,Pt-SFM 20 h电池保持稳定的性能,电压降解率仅为0.67 mV·h−1。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chinese Journal of Chemical Engineering
工程技术-工程:化工
CiteScore
6.60
自引率
5.30%
发文量
4309
审稿时长
31 days
期刊介绍:
The Chinese Journal of Chemical Engineering (Monthly, started in 1982) is the official journal of the Chemical Industry and Engineering Society of China and published by the Chemical Industry Press Co. Ltd. The aim of the journal is to develop the international exchange of scientific and technical information in the field of chemical engineering. It publishes original research papers that cover the major advancements and achievements in chemical engineering in China as well as some articles from overseas contributors.
The topics of journal include chemical engineering, chemical technology, biochemical engineering, energy and environmental engineering and other relevant fields. Papers are published on the basis of their relevance to theoretical research, practical application or potential uses in the industry as Research Papers, Communications, Reviews and Perspectives. Prominent domestic and overseas chemical experts and scholars have been invited to form an International Advisory Board and the Editorial Committee. It enjoys recognition among Chinese academia and industry as a reliable source of information of what is going on in chemical engineering research, both domestic and abroad.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


