Hydrogen generation via thermochemical decomposition of lithium hydride nano-particle in the presence of water: A temperature-accelerated reactive molecular dynamics and density functional theory based study
IF 8.3
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Understanding the hydrogen generation mechanism at the atomic length scale is critical for evolving clean energy technologies. Lithium hydride (Li–H) is a promising hydrogen storage and generation material due to its high hydrogen content and strong reactivity with water. In this paper, we have studied the formation of Li–H, its thermal stability, and the hydrogen generation during its hydrolysis, using reactive molecular dynamics simulations. Further, we employed density functional theory to obtain energy of frontier molecular orbitals to reveal the mechanisms of hydrogen formation. During hydrolysis of LiH nano-particle, the dissociation of Li–H bonds and the formation of hydrogen is observed by the adsorption of water molecules onto the Li atom of Li–H nano-particle. Further, the effect of Li–H nanoparticle size is examined using the Johnson-Mehl-Avrami-Kolmogorov (JMAK) model, and it is found that smaller nanoparticles show greater reactivity and faster hydrogen formation. Quantum chemical calculations show the reduction in HOMO-LUMO energy gap from 4.863 eV (LiH) to 4.309 eV (LiH + H2O), indicating increase in the chemical reactivity upon addition of water. In the final step, we have extended our study and observed the evolution of core-shell morphology in Li–H nanoparticles placed in H2O, CH4, CH3OH, NH3, and H2S environment at elevated temperature. Our integrated approach offers critical insight into the reaction mechanism and provides a theoretical basis for the design of efficient hydrogen-generating materials in sustainable energy systems.
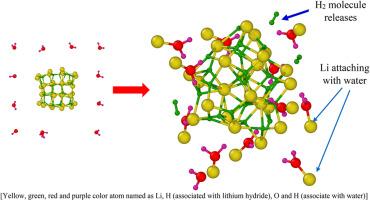
纳米氢化锂颗粒在水中热化学分解制氢:基于温度加速反应分子动力学和密度泛函理论的研究
在原子长度尺度上理解氢的产生机制对于发展清洁能源技术至关重要。氢化锂(Li-H)具有含氢量高、与水反应性强等优点,是一种很有前途的储氢和产氢材料。本文采用反应分子动力学模拟的方法研究了Li-H的形成、热稳定性和水解过程中的产氢。此外,我们利用密度泛函理论获得前沿分子轨道的能量来揭示氢的形成机制。在Li - h纳米粒子水解过程中,通过水分子吸附在Li - h纳米粒子的Li原子上,观察到Li - h键的解离和氢的形成。此外,使用Johnson-Mehl-Avrami-Kolmogorov (JMAK)模型研究了Li-H纳米颗粒尺寸的影响,发现较小的纳米颗粒具有更强的反应性和更快的氢生成速度。量子化学计算表明,HOMO-LUMO能隙从4.863 eV (LiH)减小到4.309 eV (LiH + H2O),表明加入水后化学反应活性增强。在最后一步,我们扩展了我们的研究,观察了Li-H纳米颗粒在高温下放置在H2O, CH4, CH3OH, NH3和H2S环境中的核壳形态的演变。我们的综合方法提供了对反应机制的关键见解,并为可持续能源系统中高效产氢材料的设计提供了理论基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


