Ni3Fe embedded in oxygen carriers to improve CH4 conversion and H2 production during chemical looping reforming
IF 8.3
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Chemical looping reforming is an efficient technology for the stepwise production of syngas and hydrogen, which significantly contributes to reducing carbon emissions. However, the imbalance between the catalytic performance and lattice oxygen transport rate of oxygen carriers (OCs) can induce severe carbon deposition and diminished conversion. Therefore, this study proposes a spinel OC embedded with Ni3Fe to balance them to improve conversion and reduce carbon deposition during chemical looping reforming. After H2-20 min reduction, the Ni3Fe embedded in the spinel structure generated abundant catalytic sites and suitable lattice oxygen transport rates. Moreover, increasing the reduction degree can enhance the active catalytic sites while moderating the lattice oxygen transport rate. However, over-reduction can lead to over-catalytic effect and insufficient lattice oxygen, potentially resulting in a significant carbon deposition. It is worth noting that temperature plays a more critical role in determining the lattice oxygen transport rate than the catalytic activity. Higher temperatures enhanced the lattice oxygen transport and CH4 conversion, resulting in H2/CO ratio that stabilized at 2. After 10 cycles, the CH4 conversion, H2 selectivity and CO selectivity respectively reached 94.46 %, 91.62 % and 81.63 % during CH4 conversion step, while H2 production reached 28.58 mol/h/kg during the water splitting step and carbon deposition remained below 1.79 %. The reaction kinetic showed that the reaction model of the Ni3Fe embedded OC with CH4 was Phase boundary-controlled model Infinite slabs, which facilitated the catalytic cracking of methane and reduced the activation energy of the reaction.
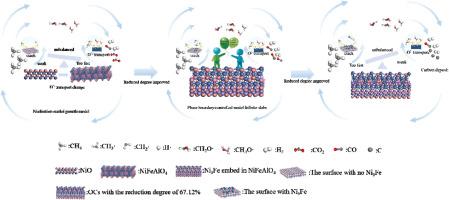
Ni3Fe嵌入氧载体提高化学环重整过程中CH4转化率和H2产量
化学环重整是一种高效的分步生产合成气和氢气的技术,对减少碳排放有重要贡献。然而,氧载体(OCs)的催化性能和晶格氧传输速率之间的不平衡会导致严重的碳沉积和转化率降低。因此,本研究提出了一种嵌入Ni3Fe的尖晶石OC来平衡它们,以提高化学环重整过程中的转化率并减少碳沉积。h2 - 20min还原后,嵌入尖晶石结构的Ni3Fe生成了丰富的催化位点和合适的晶格氧输运速率。此外,增加还原度可以增强活性催化位点,同时减缓晶格氧传输速率。然而,过度还原会导致过度催化作用和晶格氧不足,可能导致显著的碳沉积。值得注意的是,在决定晶格氧传输速率方面,温度比催化活性起着更关键的作用。较高的温度增强了晶格氧传递和CH4转化,H2/CO比稳定在2。经过10个循环后,CH4转化阶段的CH4转化率、H2选择性和CO选择性分别达到94.46%、91.62%和81.63%,而水裂解阶段的H2产量达到28.58 mol/h/kg,碳沉积保持在1.79%以下。反应动力学结果表明,Ni3Fe包埋OC与CH4的反应模型为相边界控制模型Infinite slab,有利于甲烷的催化裂化,降低了反应的活化能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


