Influence of bioactive peptides from fermented red lentil protein isolate on gut microbiota: A dynamic in vitro investigation
IF 8.2
Q1 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
Abstract
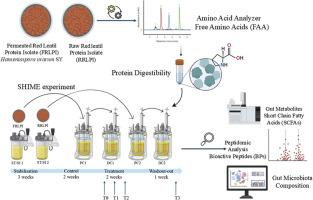
Lentils are recognized as sustainable alternative protein sources due to their favorable nutritional and functional profiles. This study evaluated the protein quality and digestibility of raw (RRLPI) and fermented (FRLPI) red lentil protein isolates and assessed their impact on the gut microbiota using the SHIME® in vitro dynamic gut model. Fermentation significantly increased in vitro protein digestibility (IVPD), from 92.5 % in RRLPI to 94.5 % in FRLPI (p < 0.05). Amino acid profiling revealed that while sulfur-containing amino acids decreased, threonine and other key essential amino acids were better preserved or enriched in FRLPI when expressed per gram of protein. Feeding with FRLPI promoted the growth of potentially beneficial colonic genera such as Lactiplantibacillus and Furfurilactobacillus and stimulated short-chain fatty acid (SCFA) production, with a notable increase in butyrate. Moreover, FRLPI digestion yielded a greater release of low-molecular-weight peptides (<3 kDa), some of which exhibited predicted antioxidant and ACE-inhibitory activities. Together, these results highlight fermentation as a strategy to optimize lentil protein functionality, improving gut microbial ecology, nutrient bioavailability, and the release of health-relevant peptides.
红豆分离蛋白发酵生物活性肽对肠道菌群影响的动态体外研究
小扁豆因其良好的营养和功能特征而被公认为可持续的替代蛋白质来源。本研究采用SHIME®体外动态肠道模型,评估了红扁豆分离物(RRLPI)和发酵(FRLPI)的蛋白质质量和消化率,并评估了它们对肠道微生物群的影响。发酵显著提高了体外蛋白质消化率(IVPD),从RRLPI的92.5%提高到FRLPI的94.5% (p < 0.05)。氨基酸谱分析显示,虽然含硫氨基酸减少,但当每克蛋白质表达时,苏氨酸和其他关键必需氨基酸在FRLPI中得到更好的保存或富集。饲喂FRLPI可促进潜在有益菌属如乳酸杆菌和呋喃乳杆菌的生长,并刺激短链脂肪酸(SCFA)的产生,丁酸显著增加。此外,FRLPI消化产生了更多的低分子量肽(< 3kda),其中一些表现出预测的抗氧化和ace抑制活性。总之,这些结果强调了发酵作为优化扁豆蛋白功能、改善肠道微生物生态、营养物质生物利用度和释放健康相关肽的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Future Foods
Agricultural and Biological Sciences-Food Science
CiteScore
8.60
自引率
0.00%
发文量
97
审稿时长
15 weeks
期刊介绍:
Future Foods is a specialized journal that is dedicated to tackling the challenges posed by climate change and the need for sustainability in the realm of food production. The journal recognizes the imperative to transform current food manufacturing and consumption practices to meet the dietary needs of a burgeoning global population while simultaneously curbing environmental degradation.
The mission of Future Foods is to disseminate research that aligns with the goal of fostering the development of innovative technologies and alternative food sources to establish more sustainable food systems. The journal is committed to publishing high-quality, peer-reviewed articles that contribute to the advancement of sustainable food practices.
Abstracting and indexing:
Scopus
Directory of Open Access Journals (DOAJ)
Emerging Sources Citation Index (ESCI)
SCImago Journal Rank (SJR)
SNIP
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


