Deciphering the risk of missense mutation rs137854486 (W1925R) of FN1 in papillary thyroid cancer: A computational and molecular dynamics approach
IF 0.7
Q4 GENETICS & HEREDITY
引用次数: 0
Abstract
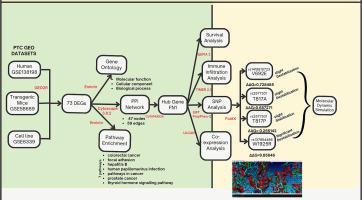
Papillary thyroid cancer (PTC) accounts for more than 85 % of all thyroid cancers. Three transcriptomic datasets of PTC, one each from humans (GSE138198), transgenic mice (GSE58689), and cell lines (GSE6339), were analysed for differentially expressed genes (DEGs). Seventy-three common DEGs were binned into significant pathways associated with cancer and immunity, for which gene ontology studies at the biological process, molecular function, and cellular component levels were performed. Protein–protein interaction (PPI) network construction and analysis of modules identified fibronectin 1 (FN1) as the critical hub gene in the pathophysiology of PTC. Survival analysis, immune infiltration analysis, and co-expression analysis of the hub genes were conducted to confirm their relationship with PTC prognosis. Analysis of four missense variants V692E, T817A, T817P and W1925R of FN1 associated with PTC, followed by structural analysis and molecular dynamics simulation, validated that the missense mutation rs137854486 (W1925R) of FN1 is significant in the tumorigenesis of PTC. FoldX predicted a significant positive ΔΔG (9.85646) value for W1925R, portraying substantial destabilization of FN1. The root mean square deviation (RMSD), root mean square fluctuation (RMSF), and radius of gyration (Rg) values of the W1925R mutant indicate significant structural deviations in the protein, resulting in increased flexibility and reduced stability. Increased flexibility, particularly in regions critical for protein function, could affect the biological activity of the protein, with a crucial influence on the pathophysiology of PTC.
解读乳头状甲状腺癌中FN1错义突变rs137854486 (W1925R)的风险:计算和分子动力学方法
乳头状甲状腺癌(PTC)占所有甲状腺癌的85%以上。分别来自人(GSE138198)、转基因小鼠(GSE58689)和细胞系(GSE6339)的3个PTC转录组学数据集分析差异表达基因(DEGs)。73个常见的deg被归类为与癌症和免疫相关的重要途径,并在生物过程、分子功能和细胞成分水平上进行了基因本体论研究。蛋白-蛋白相互作用(Protein-protein interaction, PPI)网络构建及模块分析表明,纤连蛋白1 (fibronectin, FN1)是PTC病理生理中的关键枢纽基因。通过生存分析、免疫浸润分析和枢纽基因共表达分析,证实其与PTC预后的关系。对与PTC相关的FN1的4个错义变异V692E、T817A、T817P和W1925R进行分析,并进行结构分析和分子动力学模拟,验证了FN1的错义突变rs137854486 (W1925R)在PTC的肿瘤发生中具有重要意义。FoldX预测W1925R的显著阳性ΔΔG(9.85646)值,描绘了FN1的大量不稳定。W1925R突变体的均方根偏差(RMSD)、均方根波动(RMSF)和旋转半径(Rg)值表明,该蛋白存在明显的结构偏差,导致柔韧性增加,稳定性降低。灵活性的增加,特别是在蛋白质功能的关键区域,可能影响蛋白质的生物活性,对PTC的病理生理有重要影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Human Gene
Biochemistry, Genetics and Molecular Biology (General), Genetics
CiteScore
1.60
自引率
0.00%
发文量
0
审稿时长
54 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


