Landscape of T-cell exhaustion heterogeneity and HBV integration in virus-related HCC revealed by whole-exome, transcriptome, and single-cell sequencing
IF 7.5
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
Background & Aims
To enhance our understanding of the tumor immune microenvironment (TIME) in hepatocellular carcinoma (HCC), we investigated the heterogeneity of T-cell exhaustion and its association with HBV integrations and direct oncogenic potential in HCC.
Methods
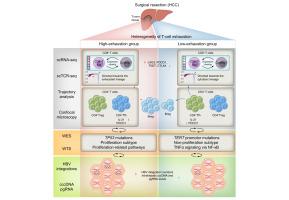
We conducted a multi-omics analysis, including single-cell RNA sequencing, whole-exome sequencing, whole-transcriptome sequencing, and next-generation sequencing (NGS)-based HBV integration analysis, in eight patients with virus-related HCC. For validation, bulk RNA sequencing and NGS-based HBV integration analysis were performed in an independent cohort (n = 106).
Results
Based on the expression scores of exhaustion markers in effector CD8+ T cells, patients were classified into high (n = 2) and low (n = 6) exhaustion groups (p <0.001). The high-exhaustion group exhibited higher clonal expansion (Gini index: 0.83 vs. 0.48, p = 0.006) and sharing of CD8+ T effector memory and cycling T cells with elevated exhaustion markers. This group also showed increased clonal expansion of CD4+ regulatory T cells and follicular helper T cells (p <0.001) with higher PDCD1 expression. In addition, the high-exhaustion group had higher TP53 mutation rates and signature scores for proliferation subtypes compared with the low-exhaustion group, who predominantly harbored TERT mutations. Moreover, the high-exhaustion group demonstrated more pronounced HBV integrations with elevated intrahepatic covalently closed circular DNA (cccDNA) and pregenomic (pg)RNA levels. Similarly, in the validation cohort, the high-exhaustion group (n = 28) demonstrated stronger proliferation subtype signatures (p <0.001), along with higher HBV integrations, S-fusion transcripts, and an increased intrahepatic viral reservoir (cccDNA/pgRNA) (p <0.05) compared with the low-exhaustion group (n = 78).
Conclusions
Our study revealed the heterogeneity in T-cell exhaustion in the TIME of HCC, along with differences in HBV integrations and molecular subtypes. These findings provide insight into the intricate relationship between high exhaustion, proliferation subtype, increased HBV integrations, and enhanced HBV-induced oncogenic potential in virus-related HCC.
Impact and implications
This study provides a comprehensive immune landscape of T-cell exhaustion using multi-omics analysis, offering critical insights into T cell heterogeneity in virus-related HCC. It establishes a strong association between higher HBV integration, enhanced oncogenic potential, T-cell exhaustion, and proliferation subtypes in HCC. Our results also establish a basis for personalized therapies tailored to the immune-exhaustion status within the TIME of each patient with HCC.
全外显子组、转录组和单细胞测序揭示了病毒相关HCC中t细胞衰竭异质性和HBV整合的景观
背景和目的为了加深我们对肝细胞癌(HCC)肿瘤免疫微环境(TIME)的理解,我们研究了t细胞耗竭的异质性及其与HBV整合和HCC直接致癌潜能的关系。方法对8例病毒相关性HCC患者进行多组学分析,包括单细胞RNA测序、全外显子组测序、全转录组测序和基于下一代测序(NGS)的HBV整合分析。为了验证,在一个独立队列(n = 106)中进行了大量RNA测序和基于ngs的HBV整合分析。结果根据效应CD8+ T细胞衰竭标志物的表达评分,将患者分为高(n = 2)和低(n = 6)衰竭组(p <0.001)。高衰竭组表现出更高的克隆扩增(基尼指数:0.83 vs. 0.48, p = 0.006),共享CD8+ T效应记忆和循环T细胞与升高的衰竭标志物。该组CD4+调节性T细胞和滤泡辅助T细胞克隆扩增增加(p <0.001), PDCD1表达升高。此外,与主要携带TERT突变的低衰竭组相比,高衰竭组具有更高的TP53突变率和增殖亚型的特征评分。此外,高衰竭组表现出更明显的HBV整合,肝内共价闭合环状DNA (cccDNA)和基因组前RNA水平升高。同样,在验证队列中,与低衰竭组(n = 78)相比,高衰竭组(n = 28)表现出更强的增殖亚型特征(p <0.001),以及更高的HBV整合、s融合转录物和增加的肝内病毒库(cccDNA/pgRNA) (p <0.05)。结论我们的研究揭示了肝癌时间中t细胞耗竭的异质性,以及HBV整合和分子亚型的差异。这些发现为了解病毒相关HCC中高度衰竭、增殖亚型、HBV整合增加和HBV诱导的致癌潜能增强之间的复杂关系提供了见解。影响和意义本研究利用多组学分析提供了T细胞耗竭的全面免疫景观,为病毒相关HCC中T细胞异质性提供了重要见解。它建立了HCC中较高的HBV整合、增强的致癌潜能、t细胞耗竭和增殖亚型之间的强烈关联。我们的研究结果还为针对每个HCC患者的免疫衰竭状态量身定制个性化治疗奠定了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

JHEP Reports
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
12.40
自引率
2.40%
发文量
161
审稿时长
36 days
期刊介绍:
JHEP Reports is an open access journal that is affiliated with the European Association for the Study of the Liver (EASL). It serves as a companion journal to the highly respected Journal of Hepatology.
The primary objective of JHEP Reports is to publish original papers and reviews that contribute to the advancement of knowledge in the field of liver diseases. The journal covers a wide range of topics, including basic, translational, and clinical research. It also focuses on global issues in hepatology, with particular emphasis on areas such as clinical trials, novel diagnostics, precision medicine and therapeutics, cancer research, cellular and molecular studies, artificial intelligence, microbiome research, epidemiology, and cutting-edge technologies.
In summary, JHEP Reports is dedicated to promoting scientific discoveries and innovations in liver diseases through the publication of high-quality research papers and reviews covering various aspects of hepatology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


