Primary percutaneous stenting for palliative biliary drainage of patients with malignant hilar biliary obstruction: TESLA trial
IF 7.5
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
Background & Aims
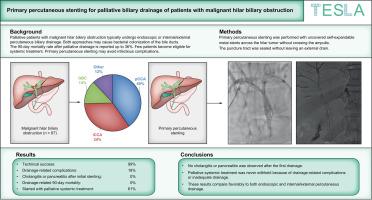
Palliative patients with malignant hilar biliary obstruction typically undergo endoscopic or internal/external percutaneous biliary drainage. Both approaches may cause bacterial colonization of the bile ducts, requiring multiple reinterventions. The 90-day mortality rate after palliative drainage is reported to be up to 36%. Few patients become eligible for systemic treatment. Primary percutaneous stenting may avoid infectious complications. The aim of this study was to investigate primary percutaneous stenting in palliative patients with malignant hilar biliary obstruction.
Methods
We performed a single-arm phase II trial. Primary percutaneous stenting was performed with uncovered self-expandable metal stents across the hilar tumor without crossing the ampulla. The puncture tract was sealed without leaving an external drain. Outcomes included drainage-related severe complications and the proportion of patients receiving systemic treatment after drainage.
Results
From October 2020 until June 2023, 67 patients were included, with perihilar cholangiocarcinoma in 27 patients (40.3%), intrahepatic cholangiocarcinoma in 23 patients (34.3%), gallbladder cancer in nine patients (13.4%), and other tumors in eight patients (12.0%). Drainage-related severe complications within 90 days were observed in 12 patients (17.9%); two patients (3.0%) developed acute cholecystitis, one patient (1.5%) had a biliary leak, three patients (4.5%) had hemorrhage, and six patients (9.0%) had persistent jaundice. No drainage-related 90-day mortality was observed. Cholangitis or pancreatitis was never observed after the first drainage. Palliative systemic treatment was started in 42 patients (62.7%).
Conclusions
Primary percutaneous stenting for patients with malignant hilar biliary obstruction had a low incidence of drainage-related complications without any cholangitis or pancreatitis after the first drainage. Palliative systemic treatment was never withheld because of drainage-related complications or inadequate drainage. These results compare favorably to both endoscopic and internal/external percutaneous drainage.
Impact and implications
This study demonstrates that primary percutaneous stenting in patients with malignant hilar biliary obstruction results in a low rate of drainage-related complications and enables initiation of systemic therapy in the majority of patients. These findings are clinically relevant for gastroenterologists, interventional radiologists, and oncologists aiming to optimize palliative care while minimizing infectious risks. The approach may offer a safe and effective alternative to conventional drainage strategies, although confirmation in comparative trials is needed to support broader implementation.
原发性经皮支架置入术治疗恶性肝门胆道梗阻患者姑息性胆道引流:TESLA试验
背景和目的姑息性恶性肝门胆道梗阻患者通常行内镜或经皮内/外胆道引流术。这两种方法都可能导致胆管细菌定植,需要多次再干预。据报道,姑息引流后90天死亡率高达36%。很少有患者有资格接受全身治疗。初次经皮支架植入术可避免感染并发症。本研究的目的是探讨恶性肝门胆道梗阻姑息性患者的原发性经皮支架植入术。方法:我们进行了一项单臂II期试验。初次经皮支架植入术是用未覆盖的自膨胀金属支架穿过肿瘤门部而不穿过壶腹。穿刺道被密封,没有留下外部引流管。结果包括引流相关的严重并发症和引流后接受全身治疗的患者比例。结果2020年10月至2023年6月,纳入67例患者,肝门周围胆管癌27例(40.3%),肝内胆管癌23例(34.3%),胆囊癌9例(13.4%),其他肿瘤8例(12.0%)。90天内出现引流相关严重并发症12例(17.9%);急性胆囊炎2例(3.0%),胆道漏1例(1.5%),出血3例(4.5%),持续性黄疸6例(9.0%)。未观察到与引流相关的90天死亡率。首次引流后未见胆管炎或胰腺炎。42例(62.7%)患者开始全身姑息治疗。结论经皮支架置入术治疗恶性肝门胆道梗阻,引流相关并发症发生率低,首次引流后无胆管炎、胰腺炎发生。姑息性全身治疗从未因引流相关并发症或引流不充分而停止。这些结果与内窥镜和内/外经皮引流比较有利。影响和意义本研究表明,恶性肝门胆道梗阻患者的原发性经皮支架置入导致引流相关并发症的发生率低,并使大多数患者能够开始全身治疗。这些发现对胃肠病学家、介入放射科医生和肿瘤学家在最小化感染风险的同时优化姑息治疗具有临床意义。该方法可能是传统排水策略的一种安全有效的替代方法,尽管需要在比较试验中得到确认,以支持更广泛的实施。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

JHEP Reports
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
12.40
自引率
2.40%
发文量
161
审稿时长
36 days
期刊介绍:
JHEP Reports is an open access journal that is affiliated with the European Association for the Study of the Liver (EASL). It serves as a companion journal to the highly respected Journal of Hepatology.
The primary objective of JHEP Reports is to publish original papers and reviews that contribute to the advancement of knowledge in the field of liver diseases. The journal covers a wide range of topics, including basic, translational, and clinical research. It also focuses on global issues in hepatology, with particular emphasis on areas such as clinical trials, novel diagnostics, precision medicine and therapeutics, cancer research, cellular and molecular studies, artificial intelligence, microbiome research, epidemiology, and cutting-edge technologies.
In summary, JHEP Reports is dedicated to promoting scientific discoveries and innovations in liver diseases through the publication of high-quality research papers and reviews covering various aspects of hepatology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


