The microenvironment modulation enabled by mass transfer control to achieve efficient CO2 electroreduction in acidic membrane electrode assembly electrolyzers
IF 7.9
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The utilization of zero-gap acidic membrane electrode assembly (MEA) electrolyzers for electrocatalytic CO2 reduction presents a significant potential for practical application, owing to their capability to effectively mitigate salt precipitation on electrodes and minimize voltage losses. However, suppressing the hydrogen evolution reaction (HER) in an H+-rich environment remains a great challenge, demanding the development of advanced catalysts and precise modulation of the microenvironment. Here, we present an efficient strategy for converting CO2 to CO using a Ni single-atom catalyst in acidic MEA-type electrolyzers. By controlling CO2 and H+ mass transfer distance to modulate the microenvironment near the catalyst, a Faradaic efficiency for CO of up to 91.3 % is achieved at 700 mA cm−2 and a pH of 1. Remarkably, the carbon utilization efficiency reaches 75.9 %, which exceeds the performance of both alkaline and neutral systems. The results reveal that, with high K+ concentration, moderate adjustment of mass transfer distance can optimize the distribution of CO2 and H+ within the catalyst layer. This optimization improves the local CO2 concentration and impedes the H+-enrichment, thereby suppressing HER during acidic CO2 electrolysis. This work may inspire further optimization of the microenvironment within the catalyst layer for electrochemical CO2 reduction in acidic electrolysis.
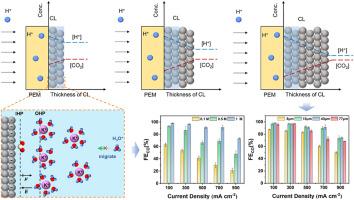
微环境调制使质传递控制实现高效的CO2电还原在酸性膜电极组装电解槽
利用零间隙酸性膜电极组件(MEA)电解槽进行电催化CO2还原具有巨大的实际应用潜力,因为它们能够有效地减轻电极上的盐沉淀并最小化电压损失。然而,在富氢环境中抑制析氢反应(HER)仍然是一个巨大的挑战,这需要开发先进的催化剂和精确的微环境调节。在这里,我们提出了一种在酸性mea型电解槽中使用Ni单原子催化剂将CO2转化为CO的有效策略。通过控制CO2和H+的传质距离来调节催化剂附近的微环境,在700 mA cm−2和pH = 1的条件下,CO的法拉第效率可达91.3%。值得注意的是,碳利用效率达到75.9%,超过了碱性和中性体系的性能。结果表明,在高K+浓度条件下,适度调节传质距离可以优化催化剂层内CO2和H+的分布。这种优化提高了局部CO2浓度,阻碍了H+的富集,从而抑制了酸性CO2电解过程中的HER。这项工作可能会启发进一步优化酸性电解中电化学CO2还原催化剂层内的微环境。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


