RAC1 as a novel therapeutic target for acute liver failure
IF 7.5
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
Background & Aims
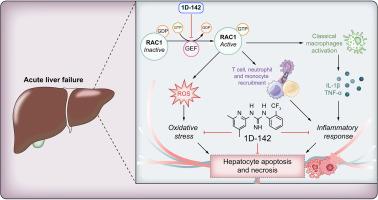
The Rho GTPase RAC1 regulates key processes in acute liver failure (ALF), including oxidative stress and inflammation. We aimed to evaluate the therapeutic potential of RAC1 inhibition in ALF.
Methods
Ingenuity Pathway Analysis and Gene Ontology analysis were performed on transcriptomic datasets from patients with ALF (GSE38941 and GSE80751). ALF was induced in mice using concanavalin A, acetaminophen, or D-galactosamine/lipopolysaccharide (n = 10-21/group). The RAC1 pharmacological inhibitor 1D-142 was used in vivo and in vitro. Hepatocytes and macrophages, from primary cultures and cell lines, were analyzed. RNA-sequencing data from ALF mouse livers (n = 3/group) were integrated with human datasets. Human liver explants (n = 6) were treated in vitro with 1D-142.
Results
RAC1 emerged as an upstream regulator correlating with immune activation and oxidative stress responses (p <0.05) in human ALF samples. Administration of 1D-142 ameliorated liver injury in murine ALF models when administered at early or late stages post-injury (p <0.05). 1D-142 treatment diminished reactive oxygen species formation (p <0.01), inflammatory cell migration (p <0.001), cytokine production (p <0.05) and hepatocyte death (p <0.05). Liver transcriptomics revealed that RAC1 inhibition modulated key dysregulated pathways in ALF. Human ALF liver explants treated with 1D-142 showed reduced necrosis (p <0.05) and reduced expression of pro-inflammatory genes (p <0.01).
Conclusions
RAC1 drives sterile inflammation and oxidative stress in ALF. Its pharmacological inhibition protects against liver injury in preclinical models and human explants, supporting RAC1 as a potential therapeutic target in ALF.
Impact and implications
Acute liver failure (ALF) is a life-threatening condition characterized by severe inflammation and oxidative stress for which there are limited treatment options. Our study provides strong scientific justification for targeting the RAC1 protein, demonstrating that its pharmacological inhibition with 1D-142 reduces liver injury, immune cell infiltration, and oxidative damage in murine models of ALF and in human liver explants. These findings identify RAC1 as a novel therapeutic target and provide translational support for its potential clinical application in ALF. RAC1-targeted therapy merits further studies in clinical settings.
RAC1作为急性肝衰竭的新治疗靶点
背景和目的Rho GTPase RAC1调节急性肝衰竭(ALF)的关键过程,包括氧化应激和炎症。我们的目的是评估抑制RAC1在ALF中的治疗潜力。方法对ALF患者(GSE38941和GSE80751)的转录组数据进行单一性途径分析和基因本体分析。用刀豆蛋白A、对乙酰氨基酚或d -半乳糖胺/脂多糖诱导小鼠ALF (n = 10-21/组)。体内外均采用RAC1药理学抑制剂1D-142。分析原代培养和细胞系的肝细胞和巨噬细胞。将ALF小鼠肝脏(n = 3/组)的rna测序数据与人类数据集进行整合。用1D-142体外处理6例人肝移植体。结果在人ALF样品中,rac1是与免疫激活和氧化应激反应相关的上游调节因子(p <0.05)。在损伤后早期或晚期给药1D-142可改善ALF模型小鼠的肝损伤(p <0.05)。1D-142治疗降低了活性氧形成(p <0.01)、炎症细胞迁移(p <0.001)、细胞因子产生(p <0.05)和肝细胞死亡(p <0.05)。肝脏转录组学显示,RAC1抑制调节了ALF的关键失调通路。1D-142处理的人ALF肝外植体显示坏死减少(p <0.05),促炎基因表达减少(p <0.01)。结论rac1介导ALF无菌性炎症和氧化应激。其药理抑制作用在临床前模型和人体外植体中保护肝脏免受损伤,支持RAC1作为ALF的潜在治疗靶点。影响和意义急性肝衰竭(ALF)是一种危及生命的疾病,其特征是严重的炎症和氧化应激,治疗方案有限。我们的研究为靶向RAC1蛋白提供了强有力的科学依据,表明其与1D-142的药理学抑制可以减轻小鼠ALF模型和人肝外植体的肝损伤、免疫细胞浸润和氧化损伤。这些发现确定了RAC1是一个新的治疗靶点,并为其在ALF中的潜在临床应用提供了翻译支持。rac1靶向治疗值得在临床环境中进一步研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

JHEP Reports
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
12.40
自引率
2.40%
发文量
161
审稿时长
36 days
期刊介绍:
JHEP Reports is an open access journal that is affiliated with the European Association for the Study of the Liver (EASL). It serves as a companion journal to the highly respected Journal of Hepatology.
The primary objective of JHEP Reports is to publish original papers and reviews that contribute to the advancement of knowledge in the field of liver diseases. The journal covers a wide range of topics, including basic, translational, and clinical research. It also focuses on global issues in hepatology, with particular emphasis on areas such as clinical trials, novel diagnostics, precision medicine and therapeutics, cancer research, cellular and molecular studies, artificial intelligence, microbiome research, epidemiology, and cutting-edge technologies.
In summary, JHEP Reports is dedicated to promoting scientific discoveries and innovations in liver diseases through the publication of high-quality research papers and reviews covering various aspects of hepatology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


