Sulfur-assisted thermal reduction synthesis of MoO2/Mo4O11 materials as high-performance cathodes for aqueous zinc-ion batteries
IF 7.9
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
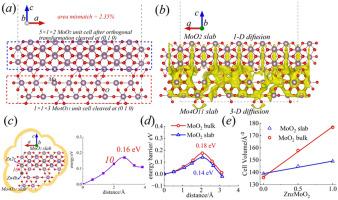
A MoO2/Mo4O11 hetero-junction material prepared by a simple sulfur-assisted thermal reduction technique as the cathode of a water-based zinc-ion battery is reported. Density functionals theories (DFT) calculations reveals that the combining of Mo4O11slab and MoO2slab aids the performance of the material in three aspects. Firstly, the facile 3D diffusion network in the case of Mo4O11slab offers the alternate Zn2+ diffusion passageway when the one dimensional diffusion channel of MoO2 is blocked either by the adsorbed S-species or the stuck Zn2+. Secondly, the formation of MoO2/Mo4O11 hetero-junction expands the interface area from MoO2 side by ca. 3.1 % and this reduces the energy barrier from Zn2c to Zn2d in the MoO2 slab to 0.14 eV. Thirdly, less volume expansion is observed in the case of MoO2 slab with the intercalation of Zn2+ compared with bulk MoO2, indicating more structural stability. Based on the synergistic effect of MoO2 and Mo4O11, the prepared MoO2/Mo4O11 hetero-junction material provides an amazing specific capacity of 260 mAh g−1 after 500 cycles at the current density of 0.5A g−1. During the long cycle (3000 cycles), the specific capacity of 184.1mAh g−1 can be maintained even if the current is as high as 2A g−1.
硫辅助热还原法制备MoO2/Mo4O11高性能锌离子电池负极材料
报道了用简单的硫辅助热还原法制备的MoO2/Mo4O11异质结材料作为水基锌离子电池正极材料。密度泛函理论(DFT)计算表明,mo4o11和MoO2slab的结合在三个方面有利于材料的性能。首先,当MoO2的一维扩散通道被吸附的s种或被吸附的Zn2+堵塞时,mo4o11板状材料中易于形成的三维扩散网络提供了交替的Zn2+扩散通道。其次,MoO2/Mo4O11异质结的形成使MoO2侧的界面面积扩大了约3.1%,使MoO2板中从Zn2c到Zn2d的能垒降低到0.14 eV。第三,与大块MoO2相比,嵌入Zn2+的MoO2板坯的体积膨胀较小,表明其结构更稳定。基于MoO2和Mo4O11的协同效应,制备的MoO2/Mo4O11异质结材料在0.5A g−1电流密度下,循环500次后的比容量达到260 mAh g−1。在长周期(3000个周期)中,即使电流高达2A g−1,也能保持184.1mAh的比容量。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


