Dual-functional palladium-cobalt nitride on exfoliated nitrogen-doped carbon nanotubes for efficient ammonia electro-oxidation in solid acid electrolysis cells toward carbon-free hydrogen production
IF 7.9
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
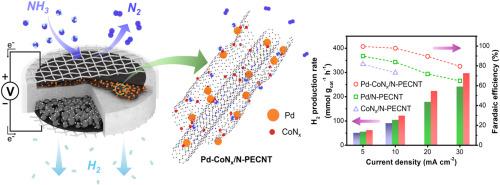
Electrochemical oxidation of gas-phase ammonia (NH3) in solid acid electrolysis cells (SAECs) is a promising approach for producing carbon-free hydrogen (H2), but its efficiency is dependent on advanced anode catalysts. This study presents a dual functional palladium–cobalt nitride supported on nitrogen doped, partially exfoliated carbon nanotubes (Pd-CoNx/N-PECNT) and systematically evaluates its performance for NH3 electrolysis in SAECs. The N-PECNT support increases electrical conductivity and basicity, stabilizes the nanoscale architecture, and facilitates electron donation to Pd-CoNx, while interfacial structure modulation strengthens adsorption and dehydrogenation pathways that govern ammonia oxidation. Relative to the comparison anode catalysts used in this study, Pd-CoNx/N-PECNT shows an H2 production rate of 296.7 mmol gcat−1 h−1 at 30 mA cm−2 and a Faradaic efficiency of 99.6 % at 10 mA cm−2, together with a lower onset potential, faster charge transfer, the lowest operating overpotential, and stable operation for 10 h. Taken together, these results indicate that Pd-CoNx/N-PECNT enables efficient, durable NH3 electrolysis in SAECs and provides a practical route for scalable H2 production.
脱片状氮掺杂碳纳米管上的双功能氮化钯钴在固体酸电解电池中高效氨电氧化以实现无碳制氢
固体酸电解电池(saec)中气相氨(NH3)的电化学氧化是一种很有前途的生产无碳氢(H2)的方法,但其效率取决于先进的阳极催化剂。本研究提出了一种双功能的钯钴氮化物,负载在氮掺杂的部分脱落碳纳米管(Pd-CoNx/N-PECNT)上,并系统地评估了其在saec中NH3电解的性能。N-PECNT载体提高了导电率和碱度,稳定了纳米级结构,促进了Pd-CoNx的电子赋能,而界面结构调节加强了控制氨氧化的吸附和脱氢途径。相对于比较在这项研究中,使用的阳极催化剂Pd-CoNx / N-PECNT显示了H2产量296.7更易gcat−1 h−1马在30厘米−2和感应电流的效率99.6%马10厘米−2,加上较低的爆发潜力,更快的电荷转移,最低的操作过电压,稳定运行10 h。总的来说,这些结果表明,Pd-CoNx / N-PECNT使高效、持久的NH3电解SAECs可伸缩的氢气生产和提供了一个实用的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


