Ultra high-rate performance LiFePO4 cathode material for next generation fast charging Li-ion batteries
IF 7.9
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
High-power cathode materials are essential for designing high-rate Li-ion batteries (LIBs), as the demand for fast charging and accelerated operation drive their growing use in applications such as electric vehicles, drones, and industrial power tools. This work investigates a high rate performing LiFePO4 (LFP) cathode material developed through a synergistic approach combining magnesium (Mg2+) ion doping, carbon coating, and the formation of micron-sized particles. Structural and morphological analyses confirm the formation of phase-pure Mg2+ doped LFP with particle size of 0.2 μm. Rietveld analysis reveals shortened P-O, Fe-O bonds, along with widened Li-O bonds upon Mg2+ doping in LiFePO4. The extended Li-O bonds are expected to significantly enhance the Li-ion kinetics even at higher current rates. These findings are further supported by density functional theory (DFT) calculations, which confirm the reduced diffusion barrier with Mg2+ doping. Electrochemical studies show that Mg2+ doped LiFePO4 delivers high-rate capability of 105 mAh/g at 20C, compared to 75 mAh/g for pristine LiFePO4, along with more than 85 % capacity retention after 800 cycles at the same rate. The enhanced high-rate performance is attributed to smaller particle size, uniform carbon coating, and widened Li-ion diffusion channels induced by Mg2+ doping in the LiFePO4 crystal structure.
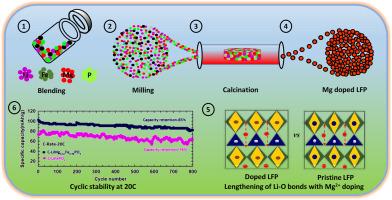
新一代快速充电锂离子电池的超高倍率性能LiFePO4正极材料
高功率正极材料对于设计高倍率锂离子电池(lib)至关重要,因为对快速充电和加速操作的需求推动了它们在电动汽车、无人机和工业电动工具等应用中的应用日益增长。本文研究了一种高性能LiFePO4 (LFP)正极材料,该材料是通过镁离子掺杂、碳涂层和微米级颗粒形成的协同方法开发的。结构和形态分析证实形成了粒径为0.2 μm的相纯Mg2+掺杂LFP。Rietveld分析表明,在LiFePO4中掺杂Mg2+后,P-O、Fe-O键缩短,Li-O键变宽。延长的Li-O键有望显著提高锂离子动力学,即使在较高的电流速率下。这些发现得到了密度泛函理论(DFT)计算的进一步支持,证实了Mg2+掺杂降低了扩散势垒。电化学研究表明,Mg2+掺杂的LiFePO4在20C下可提供105 mAh/g的高倍率容量,而原始LiFePO4的倍率为75 mAh/g,并且在相同倍率下循环800次后容量保持率超过85%。高倍率性能的增强是由于LiFePO4晶体结构中Mg2+掺杂导致的颗粒尺寸更小、碳包覆均匀以及锂离子扩散通道拓宽。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


