Tailored anchoring of FeNiOx on carbon nanotubes to enhance local electric field for boosting overall water splitting
IF 8.3
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Developing non-precious metal FeNi based bifunctional electrocatalysts for high-performance overall water splitting is crucial for advancing hydrogen energy technology. However, they commonly suffer lower activity in hydrogen evolution reaction (HER) due to unsuitable intermediates adsorption and delayed electrons transfer. In this work, by a directional arrangement strategy, raft-like FeNiOx active species were precisely constructed supported on carbon nanotubes (CNTs). This electrocatalyst exhibited significantly improving HER activity under alkaline conditions with a lower overpotential of 70 mV and Tafel slope of 51.6 mV dec−1 at current density of 10 mA cm−2. Meanwhile, it also maintained superior performance for oxygen evolution reaction (OER), requiring only 205 mV overpotential and a Tafel slope of 74.9 mV dec−1 at the same current density. A cell voltage of just 1.485 V was needed to reach 10 mA cm−2 in overall water splitting and outperformed commercial Pt/C||IrO2 electrodes. This promotion was derived from the enhanced local electronic field and electron transport on FeNiOx rafts induced by confinement effect on CNTs. Moreover, the dynamic optimization of electronic structure at Fe–O–Ni heteronuclear sites further reduced the reaction energy of rate-determining step in both HER and OER reactions. This study provides novel insights into the rational design of electrocatalyst microstructures and precise modulation of electronic properties, thereby accelerating the commercialization of overall water splitting technology.
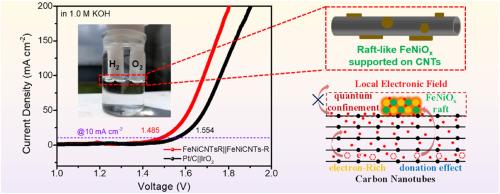
量身定制的fenix锚定在碳纳米管上,以增强局部电场,促进整体水分解
开发非贵金属FeNi基双功能电催化剂是推进氢能技术发展的关键。然而,由于中间体吸附不当和电子转移延迟,它们在析氢反应(HER)中活性较低。在这项工作中,通过定向排列策略,筏状fenix活性物质被精确地构建在碳纳米管(CNTs)上。该电催化剂在碱性条件下表现出明显的HER活性,在电流密度为10 mA cm−2时,过电位为70 mV, Tafel斜率为51.6 mV dec−1。同时,在相同的电流密度下,它也保持了良好的析氧反应(OER)性能,只需要205 mV过电位和74.9 mV dec−1的Tafel斜率。电池电压仅为1.485 V,就可以达到10 mA cm−2的整体水分解,并且优于商业Pt/C||IrO2电极。这种促进是由于碳纳米管的约束效应导致FeNiOx筏上的局部电子场和电子输运增强。此外,Fe-O-Ni异核位电子结构的动态优化进一步降低了HER和OER反应中速率决定步骤的反应能量。该研究为电催化剂微结构的合理设计和电子性质的精确调制提供了新的见解,从而加速了整体水分解技术的商业化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


