Non-invasive tests for MetALD and alcohol-related liver disease
IF 7.5
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
Metabolic- and alcohol-related liver disease (MetALD) and alcohol-related liver disease (ALD) are major drivers of the global burden of cirrhosis. While metabolic dysfunction-associated steatotic liver disease (MASLD) affects nearly one-third of the global population, ALD and MetALD, though far less common, account for a disproportionately high rate of liver-related complications and deaths. Despite this, research and clinical focus on ALD and MetALD remain limited. A critical barrier is the late stage at which these conditions are typically diagnosed, often after the onset of decompensation. In this review, we explore the potential of non-invasive tests (NITs) to change the diagnostic landscape of ALD and MetALD. NITs offer a practical and scalable means to detect liver disease at earlier, compensated stages, before symptoms emerge, thereby opening a window for timely intervention. Beyond diagnosis, these tools also serve important roles in risk assessment, disease monitoring, and evaluating treatment response. As interest in therapeutic developments for ALD and MetALD grows, NITs are expected to become central to trial design, helping to identify suitable participants, assess ongoing alcohol use, and monitor efficacy without reliance on invasive biopsies. We also discuss broader strategies necessary to support early detection, including policy changes, stigma reduction, and improved access to care. Finally, we consider emerging biomarkers and their promise in advancing precision medicine approaches tailored to this high-risk patient population.
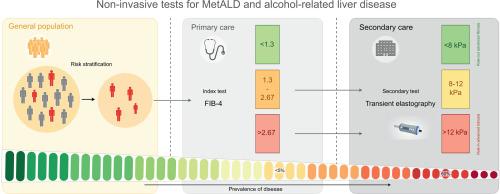
金属d和酒精相关肝病的无创检测
代谢性和酒精相关肝病(MetALD)和酒精相关肝病(ALD)是全球肝硬化负担的主要驱动因素。虽然代谢功能障碍相关的脂肪变性肝病(MASLD)影响了全球近三分之一的人口,但ALD和MetALD虽然远不常见,但却占肝脏相关并发症和死亡率的比例高得不成比例。尽管如此,对ALD和MetALD的研究和临床关注仍然有限。一个关键的障碍是这些疾病通常诊断的晚期,通常是在失代偿开始之后。在这篇综述中,我们探讨了非侵入性检查(NITs)改变ALD和MetALD诊断前景的潜力。NITs提供了一种实用且可扩展的方法,可在症状出现之前在早期的补偿阶段检测肝脏疾病,从而为及时干预打开了一扇窗。除了诊断之外,这些工具还在风险评估、疾病监测和评估治疗反应方面发挥重要作用。随着对ALD和MetALD治疗发展的兴趣日益增长,nit有望成为试验设计的核心,有助于确定合适的参与者,评估持续的酒精使用情况,并在不依赖侵入性活检的情况下监测疗效。我们还讨论了支持早期发现所需的更广泛战略,包括政策变化、减少污名和改善获得护理的机会。最后,我们考虑了新兴的生物标志物及其在推进精准医疗方法方面的前景,这些方法是为高危患者量身定制的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

JHEP Reports
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
12.40
自引率
2.40%
发文量
161
审稿时长
36 days
期刊介绍:
JHEP Reports is an open access journal that is affiliated with the European Association for the Study of the Liver (EASL). It serves as a companion journal to the highly respected Journal of Hepatology.
The primary objective of JHEP Reports is to publish original papers and reviews that contribute to the advancement of knowledge in the field of liver diseases. The journal covers a wide range of topics, including basic, translational, and clinical research. It also focuses on global issues in hepatology, with particular emphasis on areas such as clinical trials, novel diagnostics, precision medicine and therapeutics, cancer research, cellular and molecular studies, artificial intelligence, microbiome research, epidemiology, and cutting-edge technologies.
In summary, JHEP Reports is dedicated to promoting scientific discoveries and innovations in liver diseases through the publication of high-quality research papers and reviews covering various aspects of hepatology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


