Electrochemical Di-functionalization: Oxidative Amination and Oxygenation of 4-Hydroxy-α-Benzopyrones under Ring Contraction
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We report a catalyst-free, singlet oxygen-mediated electrochemical rearrangement and di-functionalization protocol for the green synthesis of biorelevant 2-hydroxy-3-oxo-2,3-dihydrobenzofuran-2-carboxamides from 4-hydroxy-α-benzopyrones. The transformation is carried out using lithium perchlorate as a cost-effective and eco-friendly electrolyte in a 1,4-dioxane:water solvent system at ambient temperature. The proposed mechanism is well supported by systematic control experiments. This one-pot strategy features a broad substrate scope, mild reaction conditions, and delivers good to excellent yields without the need for external catalysts. Key advantages of this method include operational simplicity, environmental sustainability, and high energy efficiency, making it a valuable addition to the toolbox of green synthetic methodologies.
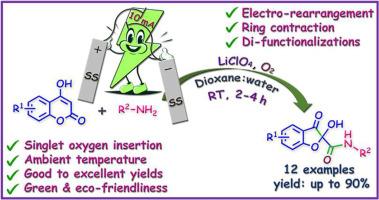
电化学双功能化:环收缩下4-羟基-α-苯并吡酮类化合物的氧化胺化和氧化
我们报道了一种无催化剂,单线态氧介导的电化学重排和双功能化方案,用于从4-羟基-α-苯并吡咯酮中绿色合成生物相关的2-羟基-3-氧-2,3-二氢苯并呋喃-2-羧酰胺。在环境温度下,在1,4-二氧六环:水溶剂体系中,使用高氯酸锂作为成本效益高且环保的电解质进行转化。该机制得到了系统控制实验的支持。这种一锅策略具有广泛的底物范围,温和的反应条件,并且在不需要外部催化剂的情况下提供良好的收率。该方法的主要优点包括操作简单,环境可持续性和高能效,使其成为绿色合成方法工具箱中有价值的补充。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


