Green chemistry advancement in methane storage: revealing the role of amino acid types and side chain characteristics in the formation and decomposition of methane hydrates
IF 7.5
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
Abstract
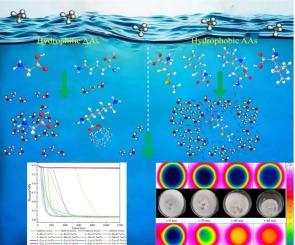
Methane hydrate (MH) plays a crucial role in energy storage, but its slow formation kinetics limits its wide application. In this study, the effects and mechanisms of six amino acids on the formation and dissociation kinetics of MH were deeply investigated around the hydrophobicity and side-chain characteristics of amino acids. The results showed that hydrophilic amino acids could not promote MH formation due to their strong interaction with H2O. On the contrary, hydrophobic amino acids could effectively promote MH formation, but their effects were affected by side-chain characteristics and concentration. In particular, methionine and phenylalanine showed the most significant promotion, with 0.5 wt% phenylalanine having a gas storage capacity of 143.02 V/V, attributed to the benzene ring structure on its side chain. Moreover, the induction time of methionine and tryptophan initially increased and then decreased with increased concentration, while the opposite trend were observed for isoleucine and phenylalanine. This phenomenon was explained by hydrophobic effects and side-chain characteristics. In terms of MH dissociation, an innovative combination of infrared thermal imaging techniques revealed similarities in the dissociation patterns of hydrophobic amino acids, but differences existed in the main dissociation stages, which were concentrated within 20–120 min and 20–60 min after thermal stimulation, respectively. This difference was mainly due to the differences in intermolecular force between side-chain structure and H2O and steric hindrance. Three consecutive experiments on the formation and dissociation of MH confirmed that methionine was better than phenylalanine in terms of stability and there was no foaming in the whole dissociation process. This study provides important theoretical support for the application of amino acids in MH.
甲烷储存中的绿色化学进展:揭示氨基酸类型和侧链特征在甲烷水合物形成和分解中的作用
甲烷水合物(MH)在能源储存中起着至关重要的作用,但其缓慢的形成动力学限制了其广泛应用。本研究围绕氨基酸的疏水性和侧链特性,深入研究了6种氨基酸对MH形成和解离动力学的影响及其机理。结果表明,亲水性氨基酸由于与水的强相互作用,不能促进MH的形成。疏水氨基酸能有效促进MH的形成,但其作用受侧链特性和浓度的影响。其中蛋氨酸和苯丙氨酸的提升最为显著,0.5 wt%苯丙氨酸由于其侧链上的苯环结构,储气容量达到143.02 V/V。蛋氨酸和色氨酸的诱导时间随浓度的增加先增加后减少,异亮氨酸和苯丙氨酸的诱导时间则相反。这种现象可以用疏水效应和侧链特性来解释。在MH解离方面,红外热成像技术的创新组合揭示了疏水氨基酸解离模式的相似性,但在主要解离阶段存在差异,分别集中在热刺激后20-120 min和20-60 min。这种差异主要是由于侧链结构与H2O分子间的作用力和位阻的差异。对MH的形成和解离进行了连续三次实验,证实蛋氨酸的稳定性优于苯丙氨酸,且在整个解离过程中没有出现泡沫。该研究为氨基酸在MH中的应用提供了重要的理论支持。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


