Investigating catalytic conversion of stearic acid to biofuels over Ni, Ag, Ti, and W supported date seed biochar under sub and supercritical conditions
IF 7.5
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
Abstract
This study presents a novel catalytic approach for converting stearic acid into biofuels using mono (Ni) and bimetallic catalysts (Ni-Ag, Ni-Ti, Ni–W) supported on date seed-derived biochar, a sustainable and cost-effective biomass waste. The reactions were carried out in a mini-reactor between 350 – 450 °C under subcritical and supercritical water conditions, without the use of hydrogen or alcohol. Catalysts were prepared via wet impregnation and characterized using BET, SEM, EDAX, and FTIR techniques. Among the catalysts, Ni-Ag/biochar achieved the highest conversion (90 %), followed by Ni–W/biochar (88 %), Ni/biochar (87 %), and Ni-Ti/biochar (80 %). Notably, product selectivity varied with temperature and catalyst composition: Temperature between 300 °C – 400 °C mainly produced unsaturated acids with minor esters, under all catalysts, through decarboxylation, esterification, and cracking reactions. Increasing the reaction temperature (at 450 °C) and changeover to supercritical water conditions enabled deeper transformations of stearic acid, generating aliphatic and aromatic hydrocarbons and FAME via decarboxylation, esterification, cyclization, epoxidation, and thermal cracking, particularly evident with Ni/biochar, Ni-Ti/biochar, and Ni-Ag/biochar. The Ni/biochar was found to be the best catalyst in producing biofuels (i.e., hydrocarbons and esters) from stearic acid, followed by Ni-Ti/biochar, and Ni-Ag/biochar. Conversely, Ni–W/biochar predominantly produced unsaturated acids from stearic acid in all temperature ranges, suggesting its inapplicability in converting stearic acid into biofuels. Thus, the work introduces a green, hydrogen-free route for biofuel production and represents the first use of date seed biochar as a catalyst support for stearic acid upgrading, offering a sustainable route for waste valorization and renewable energy generation.
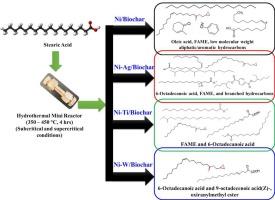
研究在亚临界和超临界条件下,硬脂酸在Ni、Ag、Ti和W支持的枣籽生物炭上催化转化为生物燃料
本研究提出了一种新的催化方法,将硬脂酸转化为生物燃料,该方法使用单(Ni)和双金属催化剂(Ni- ag, Ni- ti, Ni- w),支持枣籽衍生的生物炭,这是一种可持续且具有成本效益的生物质废物。该反应在一个小型反应器中进行,温度在350 - 450°C之间,在亚临界和超临界水条件下进行,不使用氢气或酒精。采用湿浸渍法制备了催化剂,并用BET、SEM、EDAX和FTIR技术对催化剂进行了表征。其中,Ni- ag /生物炭的转化率最高(90%),其次是Ni- w /生物炭(88%)、Ni/生物炭(87%)和Ni- ti /生物炭(80%)。值得注意的是,产物选择性随温度和催化剂组成的变化而变化:在300℃- 400℃之间,在所有催化剂下,主要通过脱羧、酯化和裂化反应生成含少量酯的不饱和酸。提高反应温度(450°C)并转换到超临界水条件下,可以使硬脂酸发生更深层次的转化,通过脱羧、酯化、环化、环氧化和热裂解生成脂肪烃、芳烃和FAME,特别是在Ni/生物炭、Ni- ti /生物炭和Ni- ag /生物炭中。Ni/生物炭是硬脂酸生产生物燃料(即碳氢化合物和酯类)的最佳催化剂,其次是Ni- ti /生物炭和Ni- ag /生物炭。相反,Ni-W /生物炭在所有温度范围内主要由硬脂酸产生不饱和酸,这表明它不适用于将硬脂酸转化为生物燃料。因此,该研究为生物燃料生产引入了一种绿色、无氢的途径,并首次使用枣籽生物炭作为硬脂酸升级的催化剂,为废物增值和可再生能源发电提供了一条可持续的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


