Integration of surfactant-modified graphene oxide with metal-organic framework-9 for enhancing chromium(VI) adsorption from aqueous media
IF 6.3
3区 工程技术
Q1 ENGINEERING, CHEMICAL
Journal of the Taiwan Institute of Chemical Engineers
Pub Date : 2025-10-04
DOI:10.1016/j.jtice.2025.106427
引用次数: 0
Abstract
Background
Chromium(VI) is a highly toxic and carcinogenic contaminant that poses significant threats to human health and environmental safety. Its effective elimination from water requires advanced treatment strategies.
Methods
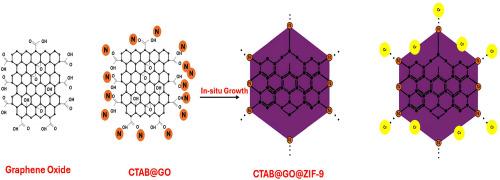
In this study, a novel nanocomposite CTAB@GO@ZIF-9 was synthesized by integrating cetyltrimethylammonium bromide (CTAB), graphene oxide (GO), and zeolitic imidazolate framework-9 (ZIF-9) to enhance Cr(VI) removal from aqueous solutions. Characterization confirmed the successful integration of components, along with improved structural uniformity and material dispersion. Response Surface Methodology (RSM) was employed to optimize the effects of adsorbent mass, initial Cr(VI) concentration, and adsorption temperature.
Significant findings
CTAB@GO@ZIF-9 demonstrated superior water stability compared to pristine ZIF-9 due to structural reinforcement by CTAB@GO. RSM optimization revealed that all investigated factors significantly influenced Cr(VI) adsorption capacity. Kinetic results followed the pseudo-second-order model (R2 = 0.9643), indicating surface-controlled adsorption via active site interactions. Freundlich isotherm (R2 = 0.9861) revealed heterogeneous adsorption, and Langmuir modeling showed a maximum capacity of 518.7 mg/g. Thermodynamic results confirmed a spontaneous and endothermic process. XPS and FTIR analyses indicated dominant nitrogen coordination from ZIF-9 and partial Cr(VI) reduction to Cr(III). Electrostatic attraction from CTAB also aided Cr(VI) uptake. These results validate the efficiency of CTAB@GO@ZIF-9 in Cr(VI) removal.
表面活性剂修饰氧化石墨烯与金属有机骨架-9的集成以增强对水介质中铬(VI)的吸附
铬(VI)是一种剧毒致癌物,对人类健康和环境安全构成重大威胁。要想从水中消除它,需要先进的处理策略。方法将十六烷基三甲基溴化铵(CTAB)、氧化石墨烯(GO)和沸石咪唑酸骨架-9 (ZIF-9)整合,合成了一种新型纳米复合材料CTAB@GO@ZIF-9,以增强水溶液中Cr(VI)的去除能力。表征证实了组件的成功集成,以及改进的结构均匀性和材料分散。采用响应面法(RSM)对吸附剂质量、初始Cr(VI)浓度和吸附温度的影响进行了优化。显著findingsCTAB@GO@ZIF-9表现出优越的水稳定性与原始ZIF-9相比,由于结构加固CTAB@GO。RSM优化结果表明,各因素对Cr(VI)吸附量均有显著影响。动力学结果符合拟二阶模型(R2 = 0.9643),表明表面控制吸附是通过活性位点相互作用实现的。Freundlich等温线(R2 = 0.9861)显示非均相吸附,Langmuir模型显示最大吸附量为518.7 mg/g。热力学结果证实了这是一个自发的吸热过程。XPS和FTIR分析表明,ZIF-9的氮配位和部分Cr(VI)还原成Cr(III)。CTAB的静电吸引也有助于Cr(VI)的吸收。这些结果验证了CTAB@GO@ZIF-9去除Cr(VI)的效率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.10
自引率
14.00%
发文量
362
审稿时长
35 days
期刊介绍:
Journal of the Taiwan Institute of Chemical Engineers (formerly known as Journal of the Chinese Institute of Chemical Engineers) publishes original works, from fundamental principles to practical applications, in the broad field of chemical engineering with special focus on three aspects: Chemical and Biomolecular Science and Technology, Energy and Environmental Science and Technology, and Materials Science and Technology. Authors should choose for their manuscript an appropriate aspect section and a few related classifications when submitting to the journal online.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


