TNF promotes osteoclastogenesis by secreting miR-31-5p into small extracellular vesicles via the autotaxin–LPA–LPAR1 axis in arthritic fibroblast-like synoviocytes
IF 3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Fibroblast-like synoviocytes (FLSs) play a crucial role in the pathogenesis of arthritis. However, the impact of small extracellular vesicles (sEVs) secreted by FLSs on osteoclastogenesis remains incompletely understood. In this study, we aimed to investigate the role of tumor necrosis factor (TNF)- and lysophosphatidic acid (LPA)-activated FLSs in sEV-mediated release of osteoclastogenic miRNAs and elucidate their functional contribution to osteoclastogenesis. Stimulation of SW982 cells with LPA or TNF significantly increased sEV secretion. TNF upregulated autotaxin expression and promoted sEV release; however, small interfering RNA (siRNA)-mediated knockdown (KD) of LPAR1 attenuated the increase in sEV release induced by the TNF–autotaxin–LPA axis. Notably, stimulation with TNF or LPA elevated syntenin-1 expression without altering its mRNA level. Furthermore, KD of the syntenin-1 gene (SDCBP) suppressed the LPA-induced increase in sEV release, indicating that syntenin-1 may mediate sEV secretion induced by the TNF–autotaxin–LPA–LPAR1 axis. sEVs derived from TNF- or LPA-treated SW982 cells stimulated osteoclastogenesis. We identified miR-31-5p as an osteoclastogenic miRNA enriched in sEVs. Expression levels of miR-31-5p in sEVs from TNF- and LPA-stimulated rheumatoid arthritis (RA) FLSs were significantly higher than in those from unstimulated RA FLSs. Treatment with a miR-31-5p mimic enhanced osteoclastogenesis by targeting large tumor suppressor kinase 2 (LATS2), whereas treatment with its inhibitor suppressed the sEV-mediated promotion of osteoclastogenesis. These findings reveal a mechanism by which TNF- and LPA-activated FLSs may facilitate sEV-mediated delivery of osteoclastogenic miRNAs, such as miR-31-5p, to osteoclast precursors, thereby contributing to osteoclast formation and bone destruction.
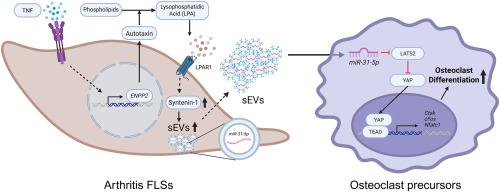
TNF通过关节炎成纤维细胞样滑膜细胞的autotaxin-LPA-LPAR1轴将miR-31-5p分泌到细胞外小泡中,从而促进破骨细胞的发生
成纤维细胞样滑膜细胞(FLSs)在关节炎的发病机制中起着至关重要的作用。然而,FLSs分泌的小细胞外囊泡(sev)对破骨细胞发生的影响仍不完全清楚。在这项研究中,我们旨在研究肿瘤坏死因子(TNF)-和溶血磷脂酸(LPA)-激活的FLSs在sev介导的破骨microrna释放中的作用,并阐明它们在破骨细胞发生中的功能贡献。LPA或TNF刺激SW982细胞可显著增加sEV分泌。TNF上调autotaxin表达,促进sEV释放;然而,小干扰RNA (siRNA)介导的LPAR1敲低(KD)减弱了TNF-autotaxin-LPA轴诱导的sEV释放的增加。值得注意的是,TNF或LPA刺激可提高syntenin-1的表达,但不改变其mRNA水平。此外,syntenin-1基因(SDCBP)的KD抑制了lpa诱导的sEV释放增加,表明syntenin-1可能介导了TNF-autotaxin-LPA-LPAR1轴诱导的sEV分泌。来自TNF-或lpa处理的SW982细胞的sev刺激了破骨细胞的发生。我们发现miR-31-5p是在sev中富集的破骨细胞microrna。TNF-和lpa刺激的类风湿关节炎(RA) FLSs的sev中miR-31-5p的表达水平显著高于未刺激的类风湿关节炎FLSs。miR-31-5p模拟物通过靶向大肿瘤抑制激酶2 (LATS2)增强破骨细胞的生成,而其抑制剂抑制sev介导的破骨细胞生成。这些发现揭示了TNF-和lpa激活的FLSs可能促进sev介导的破骨细胞microrna(如miR-31-5p)递送到破骨细胞前体的机制,从而促进破骨细胞的形成和骨破坏。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


