Fabrication of enhanced Mg2+/Li+-selective membranes via aminoacetonitrile-modulated PEI/TMC interfacial polymerization
IF 9.8
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
High-efficiency lithium extraction from salt lakes is pivotal to meet escalating lithium demand driven by energy storage and electric vehicle industries. In this study, the small-molecule aminoacetonitrile (AAN) was introduced as an aqueous-phase co-monomer to modulate the interfacial polymerization process between polyethyleneimine (PEI) and trimesoyl chloride (TMC). Specifically, the primary amine in AAN molecules reacted with acyl chloride moieties in TMC, occupying reaction sites and thereby preserving a larger fraction of unreacted primary amine groups on the PEI chains within the separation layer, which significantly enhanced the membrane's surface positive charge. Simultaneously, the nitrile groups of AAN preferentially coordinated with Li+ ions to effectively counteract the repulsive effect of the strongly positively charged membrane surface on Li+. This “charge-coordination” synergistic mechanism enabled precise control over the separation process. When treating mixed salt solution with a Mg2+/Li+ mass ratio of 100, the optimized membrane achieved a separation factor of 74.7, along with enhanced MgCl2 rejection of 99.3 % compared to virgin membrane's 93.9 %, and reduced LiCl rejection from 56.3 % to 49.3 %. Notably, upon treatment of simulated salt lake brine, the membrane manifested a pronounced Li+ enrichment effect, with a LiCl rejection rate of −8.8 % while retaining a high MgCl2 rejection capacity. This study employed a simple preparation process with industrialization potential, providing a guiding strategy for constructing high-selectivity membranes for Mg2+/Li+ separation.
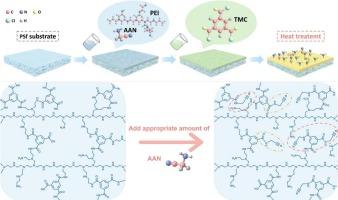
氨基乙腈调PEI/TMC界面聚合制备强化Mg2+/Li+选择性膜
从盐湖中高效提取锂对于满足储能和电动汽车行业不断增长的锂需求至关重要。在本研究中,引入小分子氨基乙腈(AAN)作为水相共聚物来调节聚乙烯亚胺(PEI)与三甲基氯(TMC)的界面聚合过程。具体来说,AAN分子中的伯胺与TMC中的酰氯基团发生反应,占据了反应位点,从而在分离层内保留了PEI链上更大比例的未反应伯胺基团,从而显著增强了膜表面的正电荷。同时,AAN的腈基优先与Li+离子配位,有效抵消了膜表面强正电荷对Li+的排斥作用。这种“电荷-配位”的协同机制能够精确控制分离过程。在处理Mg2+/Li+质量比为100的混合盐溶液时,优化后的膜分离系数为74.7,对MgCl2的截留率从原始膜的93.9%提高到99.3%,对LiCl的截留率从56.3%降低到49.3%。值得注意的是,在处理模拟盐湖卤水时,该膜表现出明显的Li+富集效果,LiCl的去除率为- 8.8%,同时保持了较高的MgCl2去除率。本研究采用了一种具有工业化潜力的简单制备工艺,为构建高选择性Mg2+/Li+分离膜提供了指导策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Desalination
工程技术-工程:化工
CiteScore
14.60
自引率
20.20%
发文量
619
审稿时长
41 days
期刊介绍:
Desalination is a scholarly journal that focuses on the field of desalination materials, processes, and associated technologies. It encompasses a wide range of disciplines and aims to publish exceptional papers in this area.
The journal invites submissions that explicitly revolve around water desalting and its applications to various sources such as seawater, groundwater, and wastewater. It particularly encourages research on diverse desalination methods including thermal, membrane, sorption, and hybrid processes.
By providing a platform for innovative studies, Desalination aims to advance the understanding and development of desalination technologies, promoting sustainable solutions for water scarcity challenges.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


