From chain length to ion diffusion: Molecular insights into phosphonium-based polymerized ionic liquid membranes for energy applications
IF 9.8
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The development of high-performance anion exchange membranes (AEMs) is essential for advanced electrochemical technologies such as alkaline fuel cells and water electrolysis. Here, we investigate the influence of phosphonium side chain length on structural hydration and chloride ion transport in polymerized ionic liquid (MPIL) membranes. Atomistic molecular dynamics simulations are conducted on hydrated MPIL systems with ethyl, butyl, and octyl n-alkyl substituents, and simulation results are validated against experimental data for water uptake and ionic conductivity.
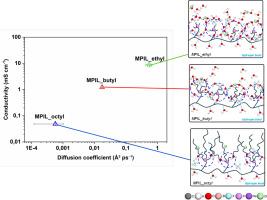
Shorter side chains (ethyl) significantly enhance water uptake (≈81 wt%) and promote the formation of interconnected hydrophilic channels, resulting in markedly higher Cl− ionic conductivity. In contrast, longer chains (octyl) restrict water accessibility and confine ion diffusion within localized hydrophilic domains, favoring ion retention tendency at the expense of transport efficiency. Intermediate chain length (butyl) yields a balanced morphology, combining moderate hydration with controllable ion mobility.
Quantitative analyses, including pore connectivity descriptors, ion–ion association free energies from RDF integration, and backbone–water interaction profiles, consistently confirm that steric hindrance modulates hydration shell formation, ion pairing, and channel percolation. This molecular-level insight suggests that alkyl chain engineering provides a tunable parameter for optimizing trade-offs between ion conductivity and relative mobility control in MPIL-based AEMs. The combined computational and experimental results provide practical guidelines for designing next-generation membranes for desalination, electrochemical conversion, and energy storage.
从链长到离子扩散:分子洞察磷基聚合离子液体膜的能源应用
高性能阴离子交换膜(AEMs)的开发对碱性燃料电池和水电解等先进的电化学技术至关重要。在这里,我们研究了磷侧链长度对聚合离子液体(MPIL)膜结构水化和氯离子传输的影响。对含有乙基、丁基和辛烷基取代基的水合MPIL体系进行了原子分子动力学模拟,并将模拟结果与水吸收和离子电导率的实验数据进行了验证。较短的侧链(乙基)显著提高了吸水性(≈81 wt%),促进了相互连接的亲水性通道的形成,导致Cl−离子电导率显著提高。相反,较长的链(辛烷基)限制了水的可及性,并限制了离子在局部亲水区域内的扩散,有利于离子的保留倾向,但牺牲了运输效率。中间链长(丁基)产生平衡的形态,结合适度的水合作用和可控的离子迁移率。定量分析,包括孔隙连通性描述符,来自RDF积分的离子-离子结合自由能,以及骨干-水相互作用剖面,一致证实了位阻调节水化壳的形成,离子配对和通道渗透。这一分子水平的见解表明,烷基链工程为优化基于mpil的AEMs中离子电导率和相对迁移率控制之间的权衡提供了一个可调参数。计算和实验结果相结合,为设计用于海水淡化、电化学转化和储能的下一代膜提供了实用指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Desalination
工程技术-工程:化工
CiteScore
14.60
自引率
20.20%
发文量
619
审稿时长
41 days
期刊介绍:
Desalination is a scholarly journal that focuses on the field of desalination materials, processes, and associated technologies. It encompasses a wide range of disciplines and aims to publish exceptional papers in this area.
The journal invites submissions that explicitly revolve around water desalting and its applications to various sources such as seawater, groundwater, and wastewater. It particularly encourages research on diverse desalination methods including thermal, membrane, sorption, and hybrid processes.
By providing a platform for innovative studies, Desalination aims to advance the understanding and development of desalination technologies, promoting sustainable solutions for water scarcity challenges.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


