Pt-based electrocatalyst for hydrogen evolution in acidic electrolytes
IF 4.2
3区 工程技术
Q2 ELECTROCHEMISTRY
引用次数: 0
Abstract
Platinum (Pt) based electrocatalysts remain the gold standard for the hydrogen evolution reaction (HER) in acidic environments due to their optimal hydrogen adsorption-free energy (ΔGH⁎ ≈ 0), high electrical conductivity, and superior chemical stability. However, the scarcity and high cost of Pt necessitate innovative strategies to reduce Pt loading while enhancing catalytic efficiency and long-term durability. This review systematically presents the recent advancements in Pt-based HER electrocatalysts, emphasizing mechanistic insights across the Volmer, Heyrovsky, and Tafel steps, and explores the influence of Pt’s electronic structure and nanostructuring on HER kinetics. Strategies such as alloying with transition metals (e.g., Ni, Co, Zn), developing single-atom catalysts (SACs), and engineering hybrid systems with supports like MXenes, graphene aerogels, and metal carbides are discussed in detail. These approaches optimize active site exposure, electronic modulation, and catalyst-support interactions to achieve high turnover frequencies, low overpotentials, and enhanced electrochemical stability under industrially relevant conditions. The review further highlights key performance indicators such as Tafel slope, mass activity, TOF, and stability, along with advanced synthesis methods, including atomic layer deposition and microwave-assisted reduction. Finally, current challenges in scalability, degradation resistance, and cost-performance trade-offs are evaluated, providing future directions toward sustainable, high-performance HER systems based on Pt. This comprehensive analysis aims to bridge the gap between fundamental catalyst design and practical hydrogen production technologies.
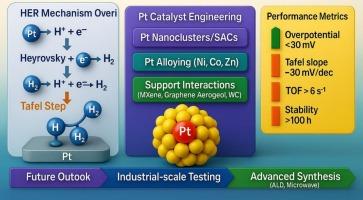
酸性电解液中析氢的pt基电催化剂
铂(Pt)基电催化剂由于其最佳的无氢吸附能(ΔGH ≈0)、高导电性和优异的化学稳定性,仍然是酸性环境下析氢反应(HER)的金标准。然而,铂的稀缺性和高成本需要创新的策略来减少铂的负载,同时提高催化效率和长期耐久性。本文系统地介绍了基于Pt的HER电催化剂的最新进展,强调了Volmer, Heyrovsky和Tafel步骤的机理见解,并探讨了Pt的电子结构和纳米结构对HER动力学的影响。本文详细讨论了过渡金属(如Ni、Co、Zn)合金化、单原子催化剂(SACs)开发以及MXenes、石墨烯气凝胶和金属碳化物等支撑材料的工程混合系统等策略。这些方法优化了活性位点暴露、电子调制和催化剂-载体相互作用,以实现高周转频率、低过电位,并在工业相关条件下增强了电化学稳定性。综述进一步强调了关键性能指标,如Tafel斜率、质量活性、TOF和稳定性,以及先进的合成方法,包括原子层沉积和微波辅助还原。最后,对当前在可扩展性、抗降解性和成本-性能权衡方面的挑战进行了评估,为基于Pt的可持续、高性能HER系统的未来发展方向提供了建议。这项综合分析旨在弥合基本催化剂设计与实际制氢技术之间的差距。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochemistry Communications
工程技术-电化学
CiteScore
8.50
自引率
3.70%
发文量
160
审稿时长
1.2 months
期刊介绍:
Electrochemistry Communications is an open access journal providing fast dissemination of short communications, full communications and mini reviews covering the whole field of electrochemistry which merit urgent publication. Short communications are limited to a maximum of 20,000 characters (including spaces) while full communications and mini reviews are limited to 25,000 characters (including spaces). Supplementary information is permitted for full communications and mini reviews but not for short communications. We aim to be the fastest journal in electrochemistry for these types of papers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


