Enhanced CO2 adsorption for hydrogen purification using calcined zeolite: Process analysis and kinetic study
IF 4.7
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
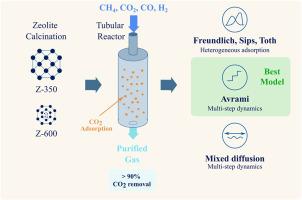
This study investigates the dynamics of CO2 adsorption on calcined zeolite at different temperatures (350 °C and 600 °C) focusing on adsorption capacity and kinetics under varying pressures and flow rates to assess its potential for industrial CO2 capture. Results show that calcination of zeolite up to 600 °C allows an increase in CO2 adsorption by increasing its porosity and mesopore content and by reducing the water content. The CO2 removal efficiency exceeds 90 %, using calcined zeolite at 600 °C, at low flow rates (0.5 L/min) and pressures higher than 3 bar. Freundlich, Sips, and Toth isotherm models described the adsorption process, indicating both monolayer and multilayer adsorption, with heterogeneous surface interactions evident. Kinetic analyses using pseudo-first-order (PFO), pseudo-second-order (PSO), and Avrami models highlighted the complexity of the adsorption mechanism. The Avrami model exhibited superior accuracy, effectively capturing multi-step and heterogeneous surface interactions. Additionally, diffusion models such as Boyd, interparticle, and Weber-Morris revealed a mixed control mechanism, with contributions from boundary layer diffusion, surface adsorption, and intraparticle diffusion.
煅烧沸石增强二氧化碳吸附净化氢气:过程分析和动力学研究
本研究研究了不同温度下(350°C和600°C)煅烧沸石对CO2的吸附动力学,重点研究了不同压力和流量下的吸附能力和动力学,以评估其在工业CO2捕集方面的潜力。结果表明,沸石煅烧至600℃时,通过增加孔隙率和介孔含量以及减少含水量,可以增加CO2的吸附量。在600°C、低流量(0.5 L/min)和高于3bar的压力下,煅烧沸石的CO2去除率超过90%。Freundlich, Sips和Toth等温线模型描述了吸附过程,表明单层和多层吸附,具有明显的非均质表面相互作用。采用伪一阶(PFO)、伪二阶(PSO)和Avrami模型进行的动力学分析突出了吸附机理的复杂性。Avrami模型表现出优异的精度,有效地捕获了多步骤和非均质表面相互作用。此外,Boyd、颗粒间和Weber-Morris等扩散模型揭示了边界层扩散、表面吸附和颗粒内扩散的混合控制机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


