Machine learning-Powered estimation of simultaneous removal of sulfamethoxazole, 17-β Estradiol, and carbamazepine via photocatalytic degradation with M-Al@ZnO
IF 7.7
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
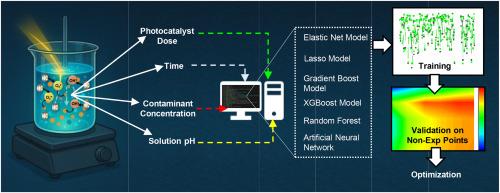
The recalcitrant nature of emerging contaminants in water has raised serious concerns, and addressing their removal aligns with the aims of SDG 6. This has necessitated research on photocatalysis, but its cost-intensive nature requires thorough optimization, which is a tedious manual process. Hence, in this study, different machine learning (ML) models (Elastic-Net, Lasso, XGBoost, Gradient Boosting (GB), Random Forest (RF), and Artificial Neural Network (ANN)) have been used to model the photocatalytic degradation of sulfamethoxazole, 17β-Estradiol, and carbamazepine in the presence of M-Al@ZnO. The training dataset included removal of the 3 contaminants, with pH varied from 2 to 10 (M-Al@ZnO dose = 0.5 g/L and contaminant concentration = 1000 μg/L), M-Al@ZnO dose varied from 0.1 to 1 g/L (pH = 8, and contaminant concentration = 1000 μg/L), and contaminant concentrations varied from 500 to 2000 μg/L (pH = 8 and M-Al@ZnO dose = 0.5 g/L). Across all the models, GB exhibited the most promising results (R2: 0.9648 and RMSE: 3.9581). SHAP analysis revealed that irradiation time (∼60–85 %) was the dominant factor, followed by pH (∼20–45 %) and dose (∼5–15 %), with pollutant concentrations having minimal or negative impact on removal. The models were then used to predict data at non-experimented points (pH varied between 2 and 10, dose varied between 0.1 and 1/g/L, and time varied between 20 and 120 min). Contour plots generated using GB-predicted data explained the interactive effects of dependent variables and hinted that at optimum conditions (pH = 8, Dose = 0.7 g/L), the system can effectively remove 90 % of contaminants within 90 min.
机器学习驱动的估计通过M-Al@ZnO光催化降解同时去除磺胺甲恶唑,17-β雌二醇和卡马西平
水中新出现的污染物的顽固性引起了严重关注,消除这些污染物符合可持续发展目标6的目标。这就需要对光催化进行研究,但它的成本高,需要进行彻底的优化,这是一个繁琐的人工过程。因此,在本研究中,不同的机器学习(ML)模型(Elastic-Net、Lasso、XGBoost、Gradient Boosting (GB)、Random Forest (RF)和Artificial Neural Network (ANN))被用于模拟M-Al@ZnO存在下磺胺甲恶唑、17β-Estradiol和卡马西平的光催化降解。训练数据集包括去除3种污染物,pH为2 ~ 10 (M-Al@ZnO剂量= 0.5 g/L,污染物浓度= 1000 μg/L), M-Al@ZnO剂量为0.1 ~ 1 g/L (pH = 8,污染物浓度= 1000 μg/L),污染物浓度为500 ~ 2000 μg/L (pH = 8,污染物浓度= 0.5 g/L)。在所有模型中,GB表现出最有希望的结果(R2: 0.9648, RMSE: 3.9581)。SHAP分析显示,辐照时间(~ 60 - 85%)是主要因素,其次是pH(~ 20 - 45%)和剂量(~ 5 - 15%),污染物浓度对去除的影响很小或有负面影响。然后使用模型预测非实验点的数据(pH值在2到10之间变化,剂量在0.1到1/g/L之间变化,时间在20到120分钟之间变化)。利用gb预测数据生成的等高线图解释了因变量的相互作用,并暗示在最佳条件下(pH = 8,剂量= 0.7 g/L),系统可以在90分钟内有效去除90%的污染物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environmental Research
环境科学-公共卫生、环境卫生与职业卫生
CiteScore
12.60
自引率
8.40%
发文量
2480
审稿时长
4.7 months
期刊介绍:
The Environmental Research journal presents a broad range of interdisciplinary research, focused on addressing worldwide environmental concerns and featuring innovative findings. Our publication strives to explore relevant anthropogenic issues across various environmental sectors, showcasing practical applications in real-life settings.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


