Exploring the role of N-S codoped carbon dots for enhanced corrosion inhibition of carbon steel in sulfuric acid
IF 4.2
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
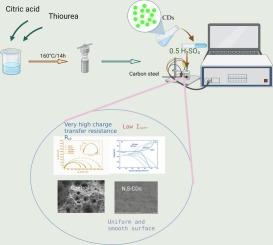
In the present work, nitrogen-doped carbon dots (N-CDs) and nitrogen-sulphur codoped carbon dots (NS-CDs) have been used as corrosion inhibitors for carbon steel in 0.5 M H2SO4. The evaluation was carried out by using weight-loss studies, electrochemical assessments, and surface morphology analyses. Although a decrease in the N content in NCDs is marked by an increase in the corrosion inhibition efficiency, the results exhibited significantly higher corrosion inhibition rates with codoped NS-CDs as compared to N-CDs, evidenced by their inhibition efficiency values (98 % vs. 88 %). In addition, NS-CDs not only revealed an increase in the charge transfer resistance value, rising from 50 to 4964 ohm cm², the corrosion current density exhibited a significant decrease (∼10−3 mA/cm²). The significant improvements were attributed to the formation of dense adsorption films and the hydrophobic properties of NS-CDs on the steel surface, leading to formation of an effective barrier against corrosion. In addition, the carbon dots that behaved as a mixed type of inhibitors (with predominant cathodic effect), were adsorbed onto the steel surface according to a Langmuir adsorption isotherm and the mechanism of inhibition is attributed to physicochemical adsorption dominated by physical interactions. The high efficiency accompanied by low corrosion current density indicates reduced corrosion rate that in turn can be beneficial in several applications, as it contributes in enhanced durability and structural sturdiness of the material.
探讨N-S共掺杂碳点增强碳钢在硫酸中的缓蚀作用
在本工作中,氮掺杂碳点(N-CDs)和氮硫共掺杂碳点(NS-CDs)作为碳钢在0.5 M H2SO4中的缓蚀剂。评估是通过失重研究、电化学评估和表面形貌分析进行的。虽然NCDs中N含量的减少会导致缓蚀效率的提高,但结果显示,共掺杂的NS-CDs的缓蚀率明显高于N- cds,缓蚀效率值(98%对88%)证明了这一点。此外,NS-CDs不仅显示出电荷转移电阻值的增加,从50上升到4964欧姆cm²,腐蚀电流密度也明显下降(~ 10−3 mA/cm²)。这一显著的改善是由于在钢表面形成了致密的吸附膜和NS-CDs的疏水性,从而形成了有效的抗腐蚀屏障。此外,碳点表现为混合类型的抑制剂(以阴极效应为主),根据Langmuir吸附等温线吸附在钢表面,其抑制机制归因于物理相互作用为主的物理化学吸附。高效率伴随着低腐蚀电流密度表明降低了腐蚀速率,这反过来在一些应用中是有益的,因为它有助于增强材料的耐久性和结构坚固性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


