Electrochemical lead detection in water using Nafion coated platinum electrodes: A sensitive approach
IF 4.2
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
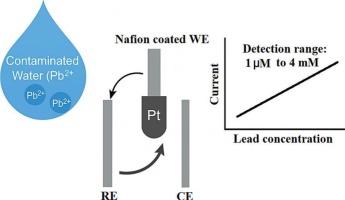
Lead is a widespread environmental pollutant with significant adverse effects on human health, particularly through contamination of drinking water and soil. The pressing need for highly sensitive, selective, and rapid detection methods has spurred extensive research across multiple disciplines. This has spurred increased interest in developing rapid and highly sensitive methods for detecting lead ions (Pb). In this paper, an innovative electrochemical sensor based on a solid-state electrolyte is introduced for lead detection in water. The sensor incorporates disposable screen-printed electrodes, including a platinum working electrode, a platinum counter electrode, and a silver reference electrode. Key performance indicators such as linearity, sensitivity, reproducibility, and detection limits were carefully evaluated within a lead concentration range of 0 mM to 4 mM. Lead concentrations were determined by monitoring current changes at varying levels. The sensor exhibited remarkable sensitivity with minimal interference from coexisting metal ions and stable performance across varying anion concentrations, and a strong linear correlation (R2 = 0.99) between current and lead concentration, with a detection limit as low as 0.5 nM. Furthermore, it demonstrated a quick response time of 1–3 s, showcasing its potential for rapid and accurate water quality monitoring.
用钠离子涂覆铂电极电化学检测水中铅:一种灵敏的方法
铅是一种广泛存在的环境污染物,特别是通过污染饮用水和土壤对人类健康产生重大不利影响。对高灵敏度、选择性和快速检测方法的迫切需求刺激了跨多个学科的广泛研究。这激发了人们对开发快速和高灵敏度检测铅离子(Pb)方法的兴趣。本文介绍了一种新型的基于固态电解质的电化学传感器,用于水中铅的检测。该传感器集成了一次性丝网印刷电极,包括铂工作电极、铂对电极和银参比电极。在0毫米至4毫米的铅浓度范围内,仔细评估了线性、灵敏度、重现性和检出限等关键性能指标。通过监测不同水平下的电流变化来确定铅浓度。该传感器具有显著的灵敏度,受共存金属离子干扰最小,在不同阴离子浓度下性能稳定,电流与铅浓度之间具有很强的线性相关性(R2 = 0.99),检测限低至0.5 nM。此外,它的快速响应时间为1-3秒,显示了它在快速准确监测水质方面的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


