Experimental and theoretical studies on the vibrational spectra and active sites of novel multisubstituted spirocycle isoindolinone-chromene hybrid derivatives
IF 4.2
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
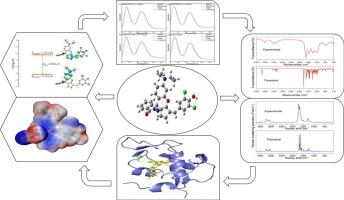
This study investigates the novel compound (Z)-2′-amino-3-(3,5-dichloro-4-hydroxybenzylidene)-6-(diethylamino)-3H-spiro[cyclopenta[b]chromene-9,1′-isoindolin]-3′-one(SPI), a spiroindolenone-chromene, through experimental and theoretical methods. Vibrational modes of SPI were identified using DFT and PED analyses, with calculated spectra matching experimental data. Frontier molecular orbital analysis revealed a HOMO-LUMO gap of 2.934 eV and ESP maps identified electrophilic regions. UV–Vis absorption spectra in various solvents were obtained and validated through TD-DFT simulations, elucidating electron transitions. Molecular docking with MDM2 protein showed strong binding (−10.72 kcal/mol) via hydrogen bonding and π-π stacking. Thermodynamic analyses indicated a transition in dynamics above 700 K, suggesting SPI's potential for drug delivery. This approach lays the groundwork for designing spiro protodrugs with tailored properties, combining spectroscopy, electronic structure theory, and biomolecular modeling.
新型多取代螺环异吲哚啉-铬杂化衍生物的振动谱和活性位点的实验与理论研究
本研究通过实验和理论方法研究了新型化合物(Z)-2′-氨基-3-(3,5-二氯-4-羟基苄基)-6-(二乙基氨基)- 3h -螺[cyclopenta[b]chromene-9,1′-isoindolin]-3′-one(SPI),一种螺吲哚酮-铬烯。利用DFT和PED分析对SPI的振动模式进行了识别,计算的光谱与实验数据相匹配。前沿分子轨道分析显示HOMO-LUMO的间隙为2.934 eV, ESP图谱确定了亲电区。通过TD-DFT模拟得到并验证了不同溶剂的紫外-可见吸收光谱,阐明了电子跃迁。与MDM2蛋白的分子对接,通过氢键和π-π堆叠表现出很强的结合(−10.72 kcal/mol)。热力学分析表明,在700 K以上的动力学转变,表明SPI具有药物传递的潜力。该方法结合了光谱学、电子结构理论和生物分子模型,为设计具有定制性质的螺旋原药奠定了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


