Transparency mechanism of egg white thermal-gels induced by molecular morphology of proteins regulated using appropriate pH
IF 11
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
The influence of morphological changes on the optical properties of proteins thermogel (TG) remains unclear, compared with the effect from charge. In this study, the variation in transparency of egg white (EW) TGs, and the changes in rheological, texture properties, microstructure, intermolecular interaction, moisture distribution and RMSD of typical protein fibers (pH2), clusters (pH4.5), dispersed spheres (pH9) and cross-linking (pH11) were performed to reveal the regulatory mechanism of molecular morphology on the transparent EW-TGs using pH. The results indicated that the formation of moderate transparent EW-TG with fibrous proteins primarily attributed to more immobile water trapped in various mesoporous and thin-walled network orderly accumulated via hydrogen bond, ionic bond and β-structure. Meanwhile, the formation of lower and higher transparent EW-TGs with spherical and ordered cross-linked proteins should mainly related to more immobile water trapped in a type of mesoporous thick-walled network assembled with β-sheet and abundant disulfide bonds, and more combined water trapped in a kind of large-pore and thick-walled network assembled with β-turn and disulfide bonds. This present work underlined the contribution of morphology to transparent EW-TG induced by appropriate pH and provided a new insight into understanding the regulatory mechanism of pH on the optical properties of protein-based TGs.
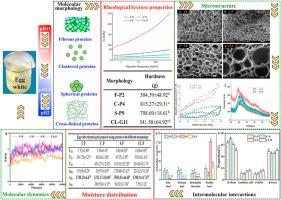
适当pH调节蛋白分子形态诱导蛋清热凝胶透明机理
与电荷的影响相比,形态变化对热凝胶(TG)光学性质的影响尚不清楚。在本研究中,研究了蛋清(EW) TGs的透明度变化,以及典型蛋白纤维(pH2)、团簇(pH4.5)、蛋白纤维的流变学、织构性能、微观结构、分子间相互作用、水分分布和RMSD的变化。通过分散球(pH9)和交联(pH11)研究了ph对透明EW-TG分子形态的调控机制。结果表明,纤维蛋白形成中等透明EW-TG主要是由于更多的不动水被困在各种介孔和薄壁网络中,通过氢键、离子键和β-结构有序积累。同时,具有球形和有序交联蛋白的低透明和高透明的ewtg的形成,主要与β-片和丰富的二硫键组装的中孔厚壁网络中捕获更多的不动水,以及β-转和二硫键组装的大孔厚壁网络中捕获更多的结合水有关。本研究强调了形态学对适当pH诱导透明EW-TG的贡献,并为理解pH对蛋白基tg光学性质的调节机制提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Hydrocolloids
工程技术-食品科技
CiteScore
19.90
自引率
14.00%
发文量
871
审稿时长
37 days
期刊介绍:
Food Hydrocolloids publishes original and innovative research focused on the characterization, functional properties, and applications of hydrocolloid materials used in food products. These hydrocolloids, defined as polysaccharides and proteins of commercial importance, are added to control aspects such as texture, stability, rheology, and sensory properties. The research's primary emphasis should be on the hydrocolloids themselves, with thorough descriptions of their source, nature, and physicochemical characteristics. Manuscripts are expected to clearly outline specific aims and objectives, include a fundamental discussion of research findings at the molecular level, and address the significance of the results. Studies on hydrocolloids in complex formulations should concentrate on their overall properties and mechanisms of action, while simple formulation development studies may not be considered for publication.
The main areas of interest are:
-Chemical and physicochemical characterisation
Thermal properties including glass transitions and conformational changes-
Rheological properties including viscosity, viscoelastic properties and gelation behaviour-
The influence on organoleptic properties-
Interfacial properties including stabilisation of dispersions, emulsions and foams-
Film forming properties with application to edible films and active packaging-
Encapsulation and controlled release of active compounds-
The influence on health including their role as dietary fibre-
Manipulation of hydrocolloid structure and functionality through chemical, biochemical and physical processes-
New hydrocolloids and hydrocolloid sources of commercial potential.
The Journal also publishes Review articles that provide an overview of the latest developments in topics of specific interest to researchers in this field of activity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


