Co-assembling de novo designed peptide with high-payload drug protein for noninvasive treatment of corneal neovascularization
IF 6.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
The specificity and low toxicity of protein drugs are significant for disease treatment but are strongly limited by their weak tissue penetrative capacity. Although formulating proteins with nanoparticle is an alternative strategy, the low encapsulation efficiency (EE) and loading capacity (LC) of protein drugs and their potential for protein inactivation remain significant challenges. Herein, the de novo designed peptide (Arg-His-Cys-Arg-His-Cys-Arg-His-Cys) (RHC)3, zinc ions (Zn2+), and the anti-neovascular protein drug Bevacizumab (Beva) were co-assembled to form PZA@Beva (peptide and Zn2+ assemblies encaspsulated Beva) nanomedicine, aiming to overcome the challenges associated with corneal neovascularization (CNV) model. The optimized size of PZA@Beva is approximately 162.5 nm, with EE% and LC% of Beva 92.7 % and 55.8 %, respectively. The bioactivity of encapsulated Beva was preserved, protecting it from proteolytic degradation, and the release of Beva from PZA@Beva exhibited pH-dependent kinetics. In vitro, PZA@Beva demonstrated effective penetration across the ocular barrier via both the paracellular pathway (by opening corneal tight junctions) and the transcellular pathway (through rapid cellular endocytosis). Additionally, PZA@Beva exhibited no cytotoxicity in vitro or in vivo, coupled with prolonged ocular retention, collectively yielding promising results for the treatment of CNV. This study contributes to non-invasive protein delivery across ocular bio-barriers for the treatment of diseases in the anterior segment.
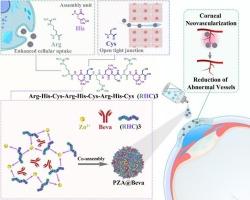
新设计肽与高负荷药物蛋白的共组装用于角膜新生血管的无创治疗
蛋白质药物的特异性和低毒性对疾病治疗具有重要意义,但其组织渗透能力弱,受到强烈限制。虽然用纳米颗粒配制蛋白质是一种替代策略,但蛋白质药物的低封装效率(EE)和负载能力(LC)及其潜在的蛋白质失活仍然是一个重大挑战。本文将从头设计的肽(Arg-His-Cys-Arg-His-Cys-Arg-His-Cys - arg - his - cys) (RHC)3、锌离子(Zn2+)和抗新生血管蛋白药物贝伐单抗(Beva)共组装形成PZA@Beva(肽和Zn2+组装包被Beva)纳米药物,旨在克服与角膜新生血管(CNV)模型相关的挑战。优化后的PZA@Beva尺寸约为162.5 nm, Beva的EE%和LC%分别为92.7%和55.8%。包裹的Beva的生物活性被保留,保护其免受蛋白水解降解,并且从PZA@Beva中释放Beva表现出ph依赖的动力学。在体外,PZA@Beva通过细胞旁通路(通过打开角膜紧密连接)和细胞外通路(通过快速细胞内吞作用)有效穿透眼屏障。此外,PZA@Beva在体外或体内均未表现出细胞毒性,并伴有长时间的眼潴留,这些都为治疗CNV提供了有希望的结果。该研究有助于通过眼生物屏障非侵入性蛋白递送治疗前段疾病。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal of Pharmaceutics: X
Pharmacology, Toxicology and Pharmaceutics-Pharmaceutical Science
CiteScore
6.60
自引率
0.00%
发文量
32
审稿时长
24 days
期刊介绍:
International Journal of Pharmaceutics: X offers authors with high-quality research who want to publish in a gold open access journal the opportunity to make their work immediately, permanently, and freely accessible.
International Journal of Pharmaceutics: X authors will pay an article publishing charge (APC), have a choice of license options, and retain copyright. Please check the APC here. The journal is indexed in SCOPUS, PUBMED, PMC and DOAJ.
The International Journal of Pharmaceutics is the second most cited journal in the "Pharmacy & Pharmacology" category out of 358 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


